Design of ROS-Triggered Sesquiterpene Lactone SC Prodrugs as TrxR1 Covalent Inhibitors for the Treatment of Non-Small Cell Lung Cancer
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
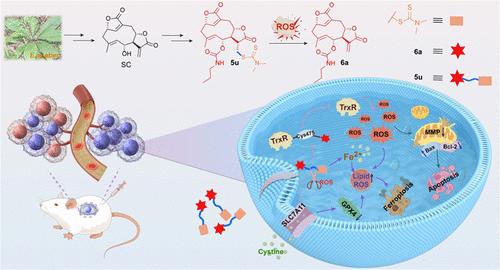
Thioredoxin reductase 1 (TrxR1) is an important therapeutic target for nonsmall cell lung cancer (NSCLC) treatment due to its overexpression in NSCLC cells. In this work, to address the deficiency that sesquiterpene lactone containing α-methylene-γ-lactone moiety was rapidly metabolized by endogenous nucleophiles, series of novel thioether derivatives were designed and synthesized based on a reactive oxygen species (ROS)-triggered prodrug strategy. Among them, prodrug 5u exhibited potent cytotoxicity against NSCLC cells and better release rates in response to ROS. The active compound 6a released from 5u covalently binds to Cys475 and Sec498 sites on TrxR1, resulting in inhibition on TrxR1 activity, which led to redox homeostasis disorder, and caused apoptosis and ferroptosis. Moreover, prodrug 5u exhibited significant antitumor efficiency in nude mice and NSCLC organoids. Our results deliver ROS-triggered prodrug 5u as a novel TrxR1 inhibitor for the treatment of NSCLC and provide a promising strategy of ROS-activated prodrug for covalent compounds in cancer therapy.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


