Nitrogen Self-Doped Graphitic Carbon Nitride with Optimized Band Gap as a Visible-Light-Driven Photocatalyst for Hydrogen Production and Pollutant Degradation
IF 3.8
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
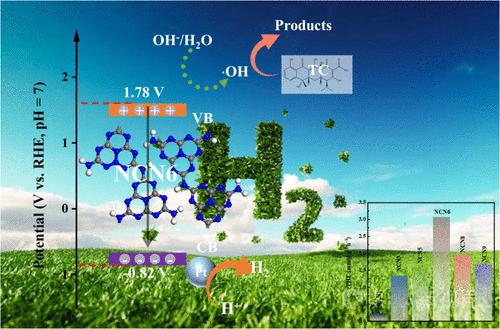
The photocatalytic activity of graphitic carbon nitride (g-C3N4) is always constrained by rapid carrier recombination, a low specific surface area, and narrowed light response. In this study, nitrogen self-doped g-C3N4 (NCN) with optimized band structure was successfully fabricated by the copolymerization of a mixture including melamine and ethylenediamine. The resultant samples were perfectly applied to the photocatalytic hydrogen production and photodegradation of tetracycline. Characterizations and density functional theory (DFT) calculations identified the introduction of a nitrogen doping site within the g-C3N4 framework. The narrowed band gap, limited photoinduced carrier recombination, and rapid charge transfer were attributed to the DFT, optical properties, and photoelectrochemical analysis, which improved the visible light absorption and optimized the carrier migration channel. Specifically, NCN6 exhibited a 13.47 times higher hydrogen evolution rate (3.07 mmol g–1 h–1) with an apparent quantum yield of 12.65% at 420 nm and 1.78-fold (82.49%) better photodegradation efficiencies than pristine g-C3N4 (0.23 mmol g–1 h–1 and 58.79%), respectively. This study offers a straightforward approach to the synthesis of functionalized g-C3N4, with the objective of enhancing the photocatalytic activity and elucidating the doping mechanism. Furthermore, it provides guidance for the development of nonmetal-doped organic semiconductor photocatalysts.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


