A novel calcination-quenching synthesis of octahedral MgO crystals with highly exposed (111) facets for enhanced adsorption performance
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
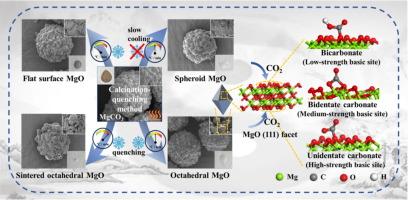
The improvement of the CO2 adsorption capacity of MgO is necessary for carbon capture. The (111) facet of MgO has garnered considerable attention recently due to its distinctive atomic arrangement of oxygen and magnesium. Theoretically, the (111) facet exposes a high density of low-coordination oxygen anions on MgO, thereby providing abundant active sites for CO2 adsorption. However, the controlled synthesis of MgO crystals with highly exposed (111) facets remains a significant challenge. In this study, a facile calcination-quenching method was developed to synthesize octahedral MgO crystals with preferentially exposed (111) facets. The effects of calcination temperature, calcination time, and cooling rate on the morphological evolution of MgO crystals were systematically investigated by using anhydrous magnesium carbonate (MgCO3) as the precursor. X-ray diffraction (XRD) results indicated that the octahedral MgO crystals preferentially expose (111) facets compared to other morphologies. Thermogravimetric analysis (TGA) reveals that the octahedral MgO exhibits superior CO2 adsorption capacity of 2.15 mmol/g at 50 °C. This value represents enhancements of 525.0 %, 161.5 %, and 144.8 % compared to commercial MgO, flat-surfaced MgO, and spherical MgO, respectively. The fundamental reasons for the superior CO2 adsorption capability of the MgO (111) facet were elucidated through in situ DRIFTS, CO2-TPD, and DFT calculations. The presence of unique low-coordinated oxygen anions on the MgO (111) facet results in enhanced Lewis basicity, thereby exhibiting a stronger binding affinity towards CO2 compared to other facets of MgO. This study provides new insights for synthesizing MgO crystals with highly (111) facets and enhanced CO2 capture capabilities.

求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


