Synergistic effects of quaternary ammonium compounds and antibiotics on the evolution of antibiotic resistance
IF 12.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
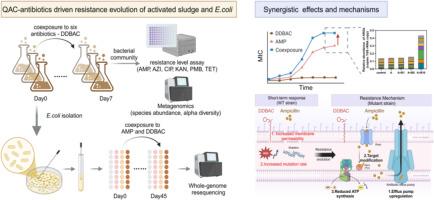
The usage of quaternary ammonium compounds (QACs) as disinfectants has surged dramatically during the COVID-19 pandemic and thereafter. QACs can promote antimicrobial resistance, but the combined effects of QACs and antibiotics in driving resistance evolution were yet revealed. This study aimed to evaluate antibiotic resistance of wastewater microorganisms under coexposure to typical antibiotics and the most widely used QAC, dodecyl dimethyl benzyl ammonium chloride (DDBAC). DDBAC exhibited synergistic effects with multiple antibiotics (ampicillin, azithromycin, ciprofloxacin, kanamycin, polymyxin B) in enhancing activated sludge resistance by 1.53–6.67 folds, compared with antibiotics exposure alone. DDBAC-ampicillin coexposure enriched multidrug and aminoglycoside ARGs with relatively high horizontal gene transfer potential. The synergistic mechanism was further explored using sludge-isolated pathogenic E. coli. DDBAC at 1–10 mg/L alone did not induce notable resistance, but synergized with ampicillin on enhancing resistance by 6.56–22.90 folds. Based on mutation analysis and transcriptomics, DDBAC-enhanced resistance evolution was attributable to efflux pump upregulation, target modification, and inhibition of ATP synthesis (a less reported mechanism). Five DDBAC-induced, resistance-conferring mutant genes were highly enriched in globally collected E. coli strains from wastewater outflow (n = 537) than soil/sediments (n = 714, p < 0.05). Considering the strong adsorption and persistence of QACs, their coexistence with antibiotics poses elevated antimicrobial resistance risks, particularly in wastewater treatment systems with long solid retention time and sewage sludge applied farmland.

季铵类化合物与抗生素协同作用对抗生素耐药性演变的影响
在 COVID-19 大流行期间及其后,季铵盐化合物(QAC)作为消毒剂的使用量急剧增加。季铵盐化合物可促进抗菌药耐药性的产生,但季铵盐化合物和抗生素在推动耐药性进化方面的共同作用尚未揭示。本研究旨在评估污水微生物在同时接触典型抗生素和最广泛使用的 QAC(十二烷基二甲基苄基氯化铵,DDBAC)时的抗生素耐药性。与单独接触抗生素相比,DDBAC 与多种抗生素(氨苄西林、阿奇霉素、环丙沙星、卡那霉素、多粘菌素 B)具有协同作用,可将活性污泥的抗药性提高 1.53-6.67 倍。DDBAC-ampicillin 共同暴露富集了多药和氨基糖苷类 ARGs,具有相对较高的水平基因转移潜力。使用污泥分离的致病性大肠杆菌进一步探讨了协同机制。单独使用 1-10 毫克/升的 DDBAC 不会诱导明显的耐药性,但与氨苄西林协同作用后,耐药性增强了 6.56-22.90 倍。根据突变分析和转录组学,DDBAC 增强的耐药性演变可归因于外排泵上调、靶点修饰和 ATP 合成抑制(一种较少报道的机制)。与土壤/沉积物(n=714,p<0.05)相比,全球收集的废水外流大肠杆菌菌株(n=537)高度富集了五个 DDBAC 诱导的耐药性突变基因。考虑到 QACs 的强吸附性和持久性,它们与抗生素共存会带来更高的抗菌药耐药性风险,尤其是在固体滞留时间较长的废水处理系统和施用污泥的农田中。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


