Encorafenib, cetuximab and chemotherapy in BRAF-mutant colorectal cancer: a randomized phase 3 trial
IF 58.7
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
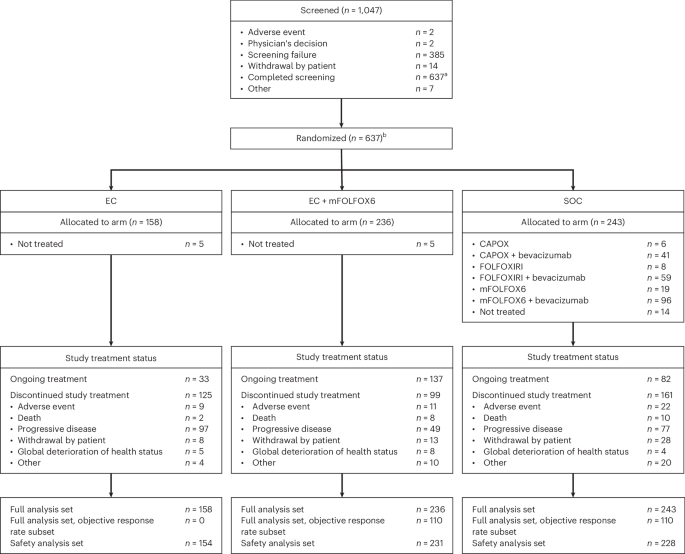
Encorafenib + cetuximab (EC) is approved for previously treated BRAF V600E-mutant metastatic colorectal cancer (mCRC) based on the BEACON phase 3 study. Historically, first-line treatment of BRAF V600E-mutant mCRC with chemotherapy regimens has had limited efficacy. The phase 3 BREAKWATER study investigated EC+mFOLFOX6 versus standard of care (SOC) in patients with previously untreated BRAF V600E mCRC. The dual primary endpoint of progression-free survival is event driven; data were not mature at data cutoff. BREAKWATER met the other dual primary endpoint of objective response rate, demonstrating significant and clinically relevant improvement in objective response rate (EC+mFOLFOX6: 60.9%; SOC: 40.0%; odds ratio, 2.443; 95% confidence interval (CI): 1.403–4.253; 99.8% CI: 1.019–5.855; one-sided P = 0.0008). Median duration of response was 13.9 versus 11.1 months. At this first interim analysis of overall survival, the hazard ratio was 0.47 (95% CI: 0.318–0.691; repeated CI: 0.166–1.322). Serious adverse event rates were 37.7% versus 34.6%. The safety profiles were consistent with those known for each agent. BREAKWATER demonstrated a significantly improved response rate that was durable for first-line EC+mFOLFOX6 versus SOC in patients with BRAF V600E mCRC. ClinicalTrials.gov identifier: NCT04607421 . As presented at the 2025 ASCO GI Cancers Symposium: in the phase 3 BREAKWATER trial, patients with previously untreated BRAF V600E metastatic colorectal cancer received the BRAF inhibitor encorafenib, the anti-EGFR monoclonal antibody cetuximab and chemotherapy mFOLFOX6 versus investigator’s choice of chemotherapy with or without bevacizumab, leading to an improved objective response rate, with the dual primary endpoint of progression free survival still maturing.

恩科非尼、西妥昔单抗和化疗治疗braf突变型结直肠癌:一项随机3期试验
基于BEACON 3期研究,enorafenib + cetuximab (EC)被批准用于先前治疗的BRAF v600e突变性转移性结直肠癌(mCRC)。从历史上看,BRAF v600e突变mCRC的一线治疗与化疗方案的疗效有限。3期BREAKWATER研究调查了EC+mFOLFOX6与先前未经治疗的BRAF V600E mCRC患者的标准护理(SOC)。无进展生存期的双重主要终点是事件驱动的;数据截止时数据尚未成熟。BREAKWATER达到了客观缓解率的另一个双重主要终点,在客观缓解率方面显示出显著的临床相关改善(EC+mFOLFOX6: 60.9%;SOC: 40.0%;优势比2.443;95%置信区间(CI): 1.403-4.253;99.8% ci: 1.019-5.855;单侧P = 0.0008)。中位反应持续时间分别为13.9个月和11.1个月。在第一次总生存率的中期分析中,风险比为0.47 (95% CI: 0.318-0.691;重复CI: 0.166-1.322)。严重不良事件发生率分别为37.7%和34.6%。安全概况与已知的每一种药物一致。在BRAF V600E mCRC患者中,与SOC相比,BREAKWATER显着提高了一线EC+mFOLFOX6的持久缓解率。ClinicalTrials.gov识别码:NCT04607421。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Medicine
医学-生化与分子生物学
CiteScore
100.90
自引率
0.70%
发文量
525
审稿时长
1 months
期刊介绍:
Nature Medicine is a monthly journal publishing original peer-reviewed research in all areas of medicine. The publication focuses on originality, timeliness, interdisciplinary interest, and the impact on improving human health. In addition to research articles, Nature Medicine also publishes commissioned content such as News, Reviews, and Perspectives. This content aims to provide context for the latest advances in translational and clinical research, reaching a wide audience of M.D. and Ph.D. readers. All editorial decisions for the journal are made by a team of full-time professional editors.
Nature Medicine consider all types of clinical research, including:
-Case-reports and small case series
-Clinical trials, whether phase 1, 2, 3 or 4
-Observational studies
-Meta-analyses
-Biomarker studies
-Public and global health studies
Nature Medicine is also committed to facilitating communication between translational and clinical researchers. As such, we consider “hybrid” studies with preclinical and translational findings reported alongside data from clinical studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


