Hybrid Approach to Predict the Effective Properties of Supercritical Carbon Dioxide Extraction Model with Linear and Nonlinear Phase Equilibrium
IF 3.8
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
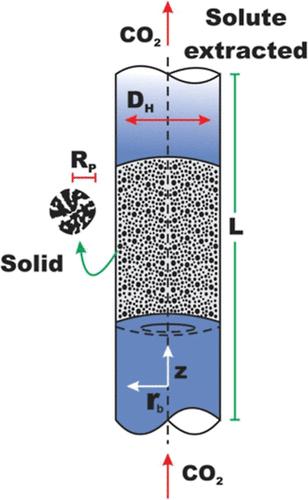
Generalized integral transform technique (GITT) is a well-established mathematical technique that can provide good results while maintaining its accuracy and reliability and eliminates the need to find an exact integral transformation, as is done by traditional analytical approaches. The coupled integrals equation approach (CIEA) is used to provide approximate relationships between mean values and potentials at the boundaries, reducing the number of independent variables. Therefore, in this present work, the GITT approach was applied to solve the two-phase extraction model with carbon dioxide as supercritical fluid by adopting both relationships, the linear Henry’s law and the nonlinear Langmuir isotherm, to describe the phase equilibrium state. The approach used in this study is mainly based on a hybrid solution that combines the CIEA and the GITT methodology. The present method showed excellent predictive capabilities on the effective extraction behavior. Furthermore, comparing the results to numerical ones from the method of lines (MOL) and those from experimental data available in the technical literature showed that the hybrid approach could provide good results while maintaining accuracy and reliability.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


