Hydroxyl Radical-Induced Calcium Phosphate Crystallization to Gain Phosphorus Fertilizers from Circulating Water
IF 7.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
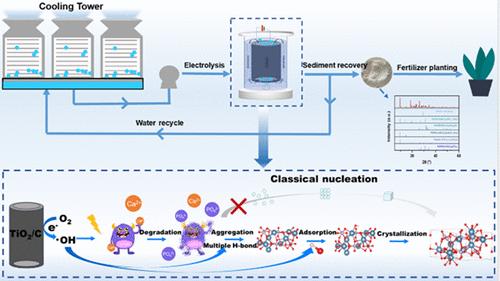
Calcium phosphate (CaP) crystallization enables simultaneous calcium removal and phosphorus recovery during circulating water (CW) treatment. Adjusting the thermodynamic process is crucial for accelerating CaP crystallization and enhancing the energy efficiency. In this study, hydroxyl radicals (·OH) generated via an electro-Fenton-like system were employed to induce CaP crystallization. Experimental investigation and theoretical calculations revealed ·OH to be key for phosphate release and CaP crystallization. The interactions between ions and NTMP are effectively strengthened by ·OH-induced multiple hydrogen bonds, leading to intermolecular aggregation and the formation of amorphous clusters. These clusters can create thermodynamic conditions that are more conducive to crystallization. Therefore, ·OH-induced CaP crystallization predominantly proceeded through the formation of amorphous clusters as the intermediate state, representing a nonclassical crystallization process. The issue of electrode deactivation caused by surface scaling was effectively avoided when crystallization primarily occurred within the amorphous clusters, resulting in the formation of CaP crystals in bulk solution. The recovered CaP crystals showed the possibility of being used as phosphorus fertilizers based on plant experiments. Finally, ·OH-induced CaP crystallization was achieved during the authentic CW treatment. This research expanded the application of ·OH to CaP crystallization and provided a sustainable strategy for CW treatment.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


