Indirect oxidation of an anionic surfactant presents in produced water in a tubular photoelectrochemical reactor with concentric expanded meshes: Experimental study and mathematical modeling
IF 5.5
3区 材料科学
Q1 ELECTROCHEMISTRY
引用次数: 0
Abstract
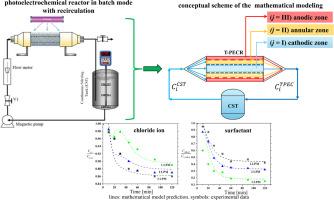
Reactive chlorine species, including chlorine (HOCl/OCl–), chlorine dioxide (ClO2), chlorine atom (Cl•), and dichlorine radical (Cl2•–), are potent oxidants with extensive water disinfection applications. This work investigated an indirect photoelectrochemical (PEC) oxidation in the presence of chloride ion (CCl-) of anionic surfactants (Shell Chemicals ENORDET™ 0242) present in oilfield-produced water. Expanded meshes of S, N-TiO2-coated titanium (outer electrode), and nickel-plated stainless steel (inner electrode) were used as photoanode and cathode, respectively in a Tubular photoelectrochemical reactor (T-PECR). The reactor was operated at different volumetric flow rates (1.0, 2.0, and 3.0 L min-1) under potentiostatic conditions (0.5 V vs. Ag/AgCl) in the presence of 50 ppm ENORDET™ 0242. A transient parametric model was developed, combining the plug dispersion exchange model (PDEM) coupled with a continuous stirred tank (CST) reactor approach. The kinetic model integrates the Gärtner-Butler framework to account for light absorption and its effect on the generation of vacancy voids (hs⁺), which drives the formation of radical productions (OH• and Cl•), and chlorine-based oxidants (Cox) for indirect surfactant degradation (CMBAS). At higher flow rates, the reduction in residence time shifts the degradation predominantly to the recirculation tank, emphasizing the indirect degradation mechanism. This trend is consistent with the observed increase in Cox concentration. Experimental results confirm that the model accurately predicts the behavior of the CCl- and CMBAS, hs+, OH•, and Cox, as functions of volumetric flow rate. The T-PECR achieved 90 % degradation of ENORDET™ 0242 in 120 min at a flow rate of 1.0 L min-¹, demonstrating the effectiveness of this approach in treating produced water. This study highlights the potential of PEC systems for efficient surfactant removal and provides insight into the interplay between reaction mechanisms and operating conditions.

求助全文
约1分钟内获得全文
求助全文
来源期刊

Electrochimica Acta
工程技术-电化学
CiteScore
11.30
自引率
6.10%
发文量
1634
审稿时长
41 days
期刊介绍:
Electrochimica Acta is an international journal. It is intended for the publication of both original work and reviews in the field of electrochemistry. Electrochemistry should be interpreted to mean any of the research fields covered by the Divisions of the International Society of Electrochemistry listed below, as well as emerging scientific domains covered by ISE New Topics Committee.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


