High entropy oxide for peroxydisulfate activation: Collaboration between oxygen vacancies and pore structure
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
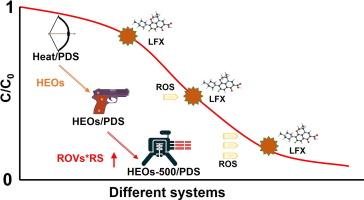
The activation of peroxydisulfate (PDS) with polymetallic components and entropy-stabilized structures of high-entropy oxides (HEOs) to eliminate organic pollutants is becoming increasingly attractive. In this paper, the orthogonal HEOs-(FeCuMnCoNi)Ox of Pbam (55) space group has been proven to be an excellent catalyst for PDS activation. By stepwise regulating the synthesis of precursors, HEOs-500 with abundant oxygen vacancies can be formed by calcining the precursors at 500 °C for 2 h. The solid solution properties of HEOs-500 result in a low total ion leaching (0.289 mg/L). The lower calcination temperature (500°C) gives it a richer specific surface area (19.79 m2/g), which is 11.24 times larger than the HEOs-800 calcined at 800 °C. A method for evaluating the catalytic activity of HEOs using the product of relative content of oxygen vacancies (ROVs) and relative specific surface area (RS) is proposed. The ROVs * RS showed a significant positive correlation (R2 > 0.98) with the quasi-first order reaction constants of quinolone antibiotics degraded by the HEOs/PDS system. The high catalytic activity of HEOs was realized, which could completely degrade levofloxacin (LFX) within 60 min. The efficient adsorption and activation capacity of (Mn Co) adsorption sites on the (201) crystal face of HEOs-500 for PDS. Free radical oxidation pathways (·OH and SO4·-) and non-free radical oxidation pathways (1O2) were shown to be responsible for the rapid degradation of LFX in the HEOs-500/PDS system. This work validates the superior prospect of HEOs catalysts in activating PDS for tail water remediation.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


