Epoxidation of propylene by molecular oxygen at low temperature over AgCl-modified Ag-Cu bimetallic catalyst
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
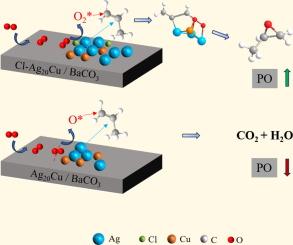
As molecular oxygen is the cheapest oxidant, the epoxidation of propylene by molecular oxygen is a topic of great interest and also the most challenge topic in the heterogeneous catalysis. However, the epoxidation of propylene by only molecular oxygen has yet to be optimised to a significant extent. Here we show, AgCl-modified Ag20Cu/BaCO3 bimetallic catalysts, prepared by in situ reduction co-deposition methods, were demonstrated to exhibit excellent catalytic activity for the epoxidation of propylene. First-principles density functional theory calculations were carried out to explore the inherent reaction mechanism and promotion effect of Cu and Cl addition. The addition of AgCl to Ag20Cu/BaCO3 bimetallic catalyst inhibited total oxidation of propylene, but enhanced both the rate of PO formation and PO selectivity. The highest PO selectivity of ∼100 % was achieved over 2400 ppm AgCl-Ag20Cu-/BaCO3 bimetallic catalyst at 80 °C.

求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


