Endoplasmic Reticulum-Targeted Polymer-Manganese Nanocomplexes for Tumor Immunotherapy
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Manganese ions (Mn2+) are an immune activator that enhances the activation of both cGAS and STING proteins. The STING signaling activation and subsequential immune responses are predominantly associated with endoplasmic reticulum (ER). Therefore, ER targeting of Mn2+ in the subcellular compartments would promote the activation of STING signaling pathways. Herein, we report the design of ER-targeted manganese-based nanocomplexes (NCs) by complexation of Mn2+ with a zwitterionic polymer, poly[2-(N-oxide-N,N-dimethylamino) ethyl methacrylate] (OPDMA). The Mn/OPDMA nanocomplexes (Mn/OPDMA NCs) keep a long blood circulation for tumor accumulation and trigger adsorption-mediated transcytosis for extravasation and deep tumor penetration. Notably, in the tumor-associated macrophages, the Mn/OPDMA NCs can preferentially translocate to their ERs, significantly enhancing cGAS-STING pathway activation for tumor-associated macrophage polarization and IFN-β secretion. In mouse colon and hepatocellular cancer models, the intravenously administrated Mn/OPDMA NCs efficiently remodel tumor immune microenvironment, greatly retard tumor growths by 2.4- to 5-fold, and prolong the mouse survivals compared to free Mn2+-treated mice. This study provides the ER-targeted delivery of Mn2+ that achieves robust STING activation and, thus, potent systemic tumor inhibition without the toxicity of free Mn2+.
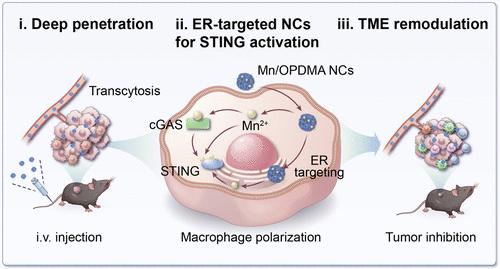
内质网靶向聚合物-锰纳米复合物用于肿瘤免疫治疗
锰离子(Mn2+)是一种免疫激活剂,可增强cGAS和STING蛋白的激活。STING信号的激活和随后的免疫反应主要与内质网(ER)有关。因此,ER靶向亚细胞区室中的Mn2+将促进STING信号通路的激活。在此,我们报道了通过将Mn2+与两性离子聚合物聚[2-(n -氧化物- n, n -二甲氨基)甲基丙烯酸乙酯](OPDMA)络合而设计的er靶向锰基纳米配合物(nc)。Mn/OPDMA纳米复合物(Mn/OPDMA NCs)为肿瘤积累保持长血液循环,并触发吸附介导的胞吞作用,使肿瘤外渗和深入渗透。值得注意的是,在肿瘤相关巨噬细胞中,Mn/OPDMA NCs可以优先转移到其内质网,显著增强cGAS-STING通路激活,促进肿瘤相关巨噬细胞极化和IFN-β分泌。在小鼠结肠癌和肝癌模型中,静脉给药的Mn/OPDMA nc有效地重塑了肿瘤免疫微环境,与游离Mn2+处理的小鼠相比,肿瘤生长明显延缓2.4- 5倍,并延长了小鼠的生存期。该研究提供了以er为靶点的Mn2+递送,实现了强大的STING激活,从而实现了有效的全身肿瘤抑制,而没有游离Mn2+的毒性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


