Beyond CO2 Storage: Enzyme-Amyloid Fibril Catalytic Hybrids for Long Cascade Reactions Converting CO2 into Fructose
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
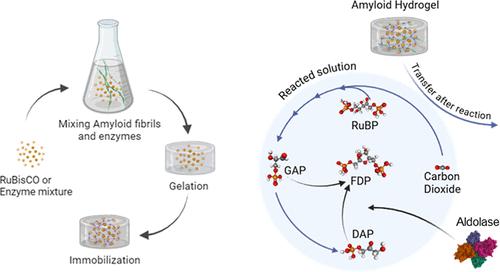
Enzyme immobilization is an efficient and cost-effective approach to recovering, stabilizing, and enhancing enzyme catalytic properties. It is a challenge, however, for coimmobilized multiple enzymes to perform consecutive reactions without being inactivated under similar conditions. Here, we present a facile enzyme immobilization platform using β-lactoglobulin amyloid fibril hydrogels. Two different hydrogels, loading either RuBisCO alone (hereby termed AFR*) or seven enzymes related to the Calvin Cycle (hereby termed AF7E hydrogel), show immobilization efficiency of over ∼95% while simultaneously exhibiting excellent activity and stability. The AFR* hydrogel enables the fixation of CO2 into 3-phosphoglycerate (3-PGA), which is then utilized as the initial step in the Calvin Cycle cascade catalytic reactions if the AF7E hydrogel is used, mimicking the light-independent part of the more complex natural photosynthesis full process. The converted substrates of this process contain precursors (α-glycerophosphate dehydrogenase and dihydroxyacetone phosphate), which can be further converted to fructose by additional aldolase. Due to the proteinaceous nature of the amyloid substrate, the AF7E hydrogel is completely biodegradable by pepsin, as confirmed via atomic force microscopy and circular dichroism spectroscopy analysis. This original enzyme-amyloid hybrid is biocompatible, sustainable, and scalable and may serve as a general template for multienzymatic catalytic platforms.
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


