CHMP4C promotes pancreatic cancer progression by inhibiting necroptosis via the RIPK1/RIPK3/MLKL pathway
IF 11.4
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
Pancreatic cancer (PC) cannot currently be completely cured and has a poor prognosis. Necroptosis is a distinct form of regulated cell death that differs from both necrosis and apoptosis. Understanding the role of necroptosis during PC progression would open new avenues for targeted therapy.Objectives
The purpose of this study is to examine the impact of necroptosis on the progression of PC and related mechanisms.Methods
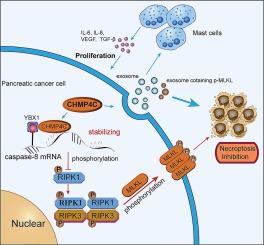
RNA sequencing was performed to identify necroptosis-related genes that are differentially expressed in PC tissues. The biological functions of CHMP4C and its necroptosis effects were determined in vitro and in vivo. RNA immunoprecipitation, MeRIP-qPCR, Co-immunoprecipitation assays were conducted to evaluate the interaction among CHMP4C, YBX1 and caspase-8 mRNA. Extracellular vesicles were isolated using the differential ultracentrifugation method. The expression of CHMP4C, p-MLKL and CD117 were detected on a PC tissue microarray using multiplex immunofluorescence staining.Results
CHMP4C was significantly overexpressed in PC cells and tissues. It promoted cell growth and suppressed necroptosis of PC cells in both in vivo and in vitro settings. Mechanistically, CHMP4C interacted with YBX1 to mediate m5C modification of caspase-8 mRNA, resulting in increased caspase-8 expression and inhibition of RIPK1/RIPK3/MLKL pathway phosphorylation. Furthermore, CHMP4C promoted extracellular exocytosis of p-MLKL to further suppress necroptosis. Additionally, PC cells used CHMP4C within extracellular vesicles to recruit and stimulate mast cells (MCs), which in turn promoted PC cell proliferation. In PC tissues, the expression of CHMP4C showed a negative correlation with p-MLKL and a positive association with CD117. High expression levels of CHMP4C in patients were associated with poorer overall survival outcomes.Conclusions
CHMP4C promotes PC progression by inhibiting necroptosis, which has potential as a biomarker and therapeutic target in PC.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


