CO2 Hydrogenation to Methanol on CoIn2/In2O3: The Role of the Alloy/Oxide Interface in Driving Catalytic Activity and Selectivity
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
CO2 catalytic hydrogenation to methanol is promising for CO2 utilization. In2O3-based catalysts are attracting much attention because of their high methanol selectivity. However, a low CO2 conversion rate limits the overall methanol yield. Herein, we developed the interface of the CoIn2 alloy and In2O3 oxide as a CoIn2/In2O3 catalyst and successfully achieved high performance for the hydrogenation of CO2 to methanol. Experimental and theoretical results indicated that the alloy/oxide interface is stable during the reaction atmosphere; the high performance arising from the electronic interaction between CoIn2 and In2O3, which improves the electron density at the CoIn2 interface, facilitates H2 dissociation, CO2 adsorption, and the hydrogenation of formate intermediates to methanol, which justifies the sustained high methanol selectivity and production rate. The optimized catalyst showed a methanol selectivity up to 74% and a high methanol space-time yield up to 0.69 gMeOHgcat–1h–1 at 5.0 MPa, H2/CO2 = 3:1, 300 °C and 36,000 mLgcat–1h–1.
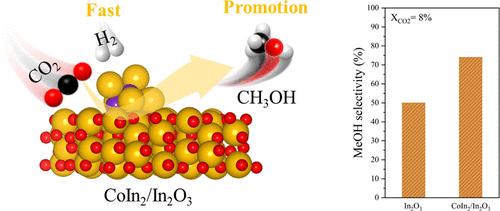
co_2 /In2O3催化CO2加氢制甲醇:合金/氧化物界面对催化活性和选择性的影响
CO2催化加氢制甲醇是CO2利用的重要途径。基于in2o3的催化剂因其高的甲醇选择性而备受关注。然而,较低的二氧化碳转化率限制了甲醇的总产量。在此,我们开发了co_2合金和In2O3氧化物的界面作为co_2 /In2O3催化剂,并成功地实现了CO2加氢制甲醇的高性能。实验和理论结果表明,在反应气氛中,合金/氧化物界面是稳定的;co_2和In2O3之间的电子相互作用提高了co_2界面上的电子密度,促进了H2的解离、CO2的吸附以及甲酸酯中间体加氢生成甲醇,从而保证了甲醇的高选择性和高收率。优化后的催化剂在5.0 MPa、H2/CO2 = 3:1、300℃、36000 mLgcat-1h-1条件下,甲醇选择性高达74%,甲醇空时产率高达0.69 gMeOHgcat-1h-1。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


