Modulation of Schottky Barrier Height and Electronic Structure in Transition-Metal@Nitrogen-Doped-Carbon Core–Shell Cocatalysts Loaded with MnxCd1–xS Nanorods for Enhanced Photocatalytic Hydrogen Evolution
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Transition-metal (TM)/carbon nanocomposites have emerged as a type of low-cost high-efficiency photocatalysis cocatalyst for their high photoactivity comparable to typical noble metals such as Pt. However, the underlying cocatalytic mechanism in this combined nanoscaled system has not been understood sufficiently. In this work, a core–shell nanoparticle (NP) cocatalyst has been designed and synthesized by using three kinds of large-work-function TMs (Co, Ni, and Cu) and nitrogen-doped carbon (NC) to construct a core–shell structure (TM@NC), and its photocatalytic hydrogen evolution performance has been studied with MnxCd1–xS nanorods (NRs). It has been found that the TMs endow the TM@NC NPs with large work functions, which act as efficient electron traps. Significantly, the Schottky barrier height at the interface of TM@NC and MnxCd1–xS can be modulated by the embedded TMs, and therefore the transfer efficiency of the photoelectrons can be adjusted. Thus, the relationships between photoactivity and the Schottky barrier height can be obtained. The NC layer in the TM@NC structure not only protects the embedded TMs from overoxidation but also provides abundant catalytic sites for hydrogen evolution reaction. Meanwhile, the electronic structure of the NC layer can be refined by the embedded TMs as well, favoring the release of hydrogen from its surface. As a result, all the TM@NC NP cocatalysts exhibit comparable cocatalytic activity to optimized Pt NPs, and the Ni@NC sample obviously outperforms Pt. This work highlights the effect of the Schottky barrier in adjusting the photocatalytic activity and provides deep insight into the photoactivity engineering of photocatalysts by incorporating TMs with semiconductor materials.
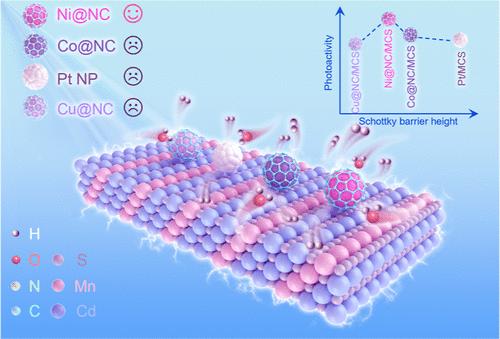
负载MnxCd1-xS纳米棒的Transition-Metal@Nitrogen-Doped-Carbon核壳共催化剂中肖特基势垒高度和电子结构的调制增强光催化析氢
过渡金属/碳纳米复合材料作为一种低成本、高效率的光催化共催化剂,具有与铂等贵金属相当的高光活性。然而,这种纳米复合体系的潜在共催化机制尚未得到充分的了解。本文采用三种大功函数TMs (Co、Ni和Cu)和氮掺杂碳(NC)构建核壳结构,设计合成了核壳纳米粒子(NP)共催化剂(TM@NC),并利用MnxCd1-xS纳米棒(NRs)研究了其光催化析氢性能。研究发现,TMs使TM@NC NPs具有较大的功函数,作为有效的电子陷阱。值得注意的是,TM@NC和MnxCd1-xS界面处的肖特基势垒高度可以被嵌入的TMs调制,从而可以调节光电子的转移效率。由此可以得到光活性与肖特基势垒高度之间的关系。TM@NC结构中的NC层不仅保护了嵌入的TMs不被过度氧化,而且为析氢反应提供了丰富的催化位点。同时,数控层的电子结构也可以通过嵌入的TMs进行细化,有利于氢从其表面释放。结果,所有的TM@NC NP共催化剂都表现出与优化后的Pt NP相当的共催化活性,并且Ni@NC样品的共催化活性明显优于Pt。本工作突出了肖特基势垒在调节光催化活性方面的作用,并通过将tm与半导体材料结合在一起,为光催化剂的光活性工程提供了深入的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


