A Set of Three GdIII Spin Labels with Methanethiosulfonyl Groups for Bioconjugation Covering a Wide Range of EPR Line Widths
IF 3.6
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Spin labels based on GdIII complexes are important tools for the elucidation of the structure, dynamics and interaction of biomolecules by electron paramagnetic resonance (EPR) spectroscopy. Their EPR spectroscopic properties line width and relaxation times influence their performance in a particular application. To be able to apply a complex well-suited for a specific application, a set of GdIII complexes with different EPR spectroscopic properties ready-made for spin labeling will be highly useful. We prepared three GdIII complexes with DO3APic, NO3Pic, and PyMTA as the basic ligand units. They cover a wide range of EPR line widths but have in common a cysteine-targeting methanethiosulfonyl (MTS) group connected to a pyridine ring, which is an intrinsic part of the ligand. The reaction with a cysteine-containing pentapeptide (0.45 mM in the peptide, pH ∼ 7) was complete within 90 s and chemoselective. The MTS group hydrolyzed with half-lives of >24, 8, 2, and 1 h at pH 5, 6, 7, and 8, respectively. The structurally related nicotinic acid-substituted disulfide (NDS) group was found to be hydrolytically much more stable. However, the MTS spin label clearly won the competition for the pentapeptide over the NDS spin label. If high reactivity is essential, MTS is clearly the better choice.
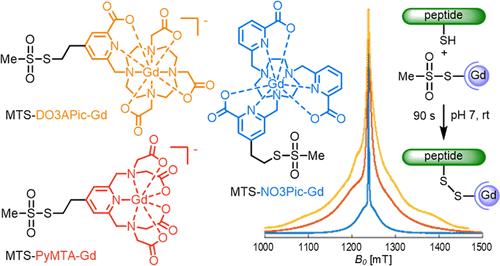
一组具有甲乙磺酰基的三种GdIII自旋标签,用于生物偶联,覆盖了大范围的EPR线宽
基于GdIII配合物的自旋标记是利用电子顺磁共振(EPR)光谱分析生物分子结构、动力学和相互作用的重要工具。它们的EPR光谱特性线宽和弛豫时间影响它们在特定应用中的性能。为了能够应用非常适合特定应用的复合物,一组具有不同EPR光谱特性的现成的用于自旋标记的GdIII复合物将非常有用。我们制备了以DO3APic、NO3Pic和PyMTA为基本配体单元的三种GdIII配合物。它们覆盖了很宽的EPR谱线宽度,但都有一个与吡啶环相连的靶向半胱氨酸的甲乙磺酰基(MTS)基团,这是配体的固有部分。与含半胱氨酸的五肽(肽中0.45 mM, pH ~ 7)的反应在90 s内完成,并且具有化学选择性。MTS组水解的半衰期分别为24、8、2和1小时,pH值分别为5、6、7和8。结构相关的烟酸取代二硫化物(NDS)基团在水解上更稳定。然而,MTS自旋标签显然比NDS自旋标签赢得了五肽的竞争。如果需要高反应性,MTS显然是更好的选择。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


