Unlocking phosphorus recovery from microalgae biomass: the enhanced transformation and release of phosphorus species
IF 11.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
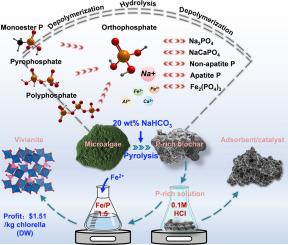
The intertwined challenges of harmful algae blooms and the phosphorus (P) resource crisis have necessitated the recovery of P from algae biomass. For the first time, a co-pyrolysis strategy that incorporates NaHCO3 into the pyrolysis process of chlorella to efficiently recover P in the form of vivianite was proposed. The findings demonstrated that the addition of 20 wt% NaHCO3 during pyrolysis significantly enhanced P extraction from biochar, increasing the extraction efficiency from 2.8% to 94.37%. A complementary array of techniques including chemical extraction, nuclear magnetic resonance (NMR) spectroscopy, fourier-transform infrared spectroscopy (FTIR) and X-ray diffraction (XRD), as well as two-dimensional correlation spectroscopy (2D-COS), was employed to elucidate the transformation of hard-to-extract P in chlorella to easy-to-extract P during pyrolysis. It was observed that organophosphorus (OP), pyrophosphate (pyro-P), and polyphosphates (poly-P) reacted with NaHCO3 at 700°C, undergoing depolymerization and hydrolysis, which led to the formation of orthophosphate (ortho-P) species (e.g., Na3PO4, NaCa(PO4)3, (Fe2(PO4)3), accounting for 98.88% of the P species in biochar product. High-purity vivianite (∼98.13%) was subsequently obtained without the need for impurity removal, as indicated by chemical equilibrium simulations, due to the minimal ions and dissolved organic matter (DOM) present in the leaching solution, a consequence of the simple and pure structure of microalgae biomass. The estimated economic profit of this strategy is $1.51 per kilogram of dry chlorella. Additionally, the resulting biochar exhibited a high surface area (518.40 m²/g) and a well-developed pore structure, make it a promising material for adsorption and catalytic applications. This study provides a novel perspective for addressing the P crisis while effectively mitigating harmful algal blooms.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


