Superior photocatalytic activity of Pt-loaded rutile-type TiO2 particles in brine splitting reaction
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
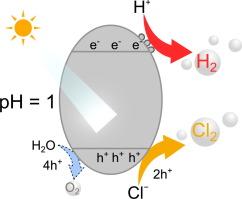
A Pt-loaded rutile-type TiO2 photocatalyst was investigated for splitting brine (aqueous NaCl solution) into hydrogen and chlorine as a new artificial photosynthesis reaction. Although most of Pt species dissolved in solution during induction period, the small amount of Pt remaining on the TiO2 surface was active and stable, resulting in continuous hydrogen and chlorine production for 40 h at a catalytically sufficient turnover number. The photocatalytic activity of Pt-loaded TiO2 increased to more than 10 times the previously reported value, and the quantum efficiency and solar energy conversion efficiency reached 4.3 % at 365 nm and 0.07 %, respectively. Notably, this activity is also higher than that reported for one-step pure water splitting reactions using TiO2-based photocatalysts. Although chlorine production is thermodynamically more challenging than the oxygen evolution reaction, chlorine production involving a two-electron oxidation process is more kinetically favorable than oxygen production involving a four-electron oxidation process, which is considered as the rate-determining step. Our findings open a new direction for brine splitting and also enrich the field of artificial photosynthetic reactions.

求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


