Design of (R)-3-(5-Thienyl)carboxamido-2-aminopropanoic Acid Derivatives as Novel NMDA Receptor Glycine Site Agonists: Variation in Molecular Geometry to Improve Potency and Augment GluN2 Subunit-Specific Activity
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
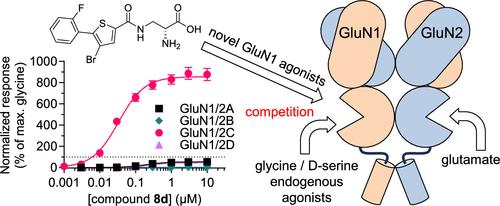
NMDA receptor ligands have therapeutic potential in neurological and psychiatric disorders. We designed (R)-3-(5-thienyl)carboxamido-2-aminopropanoic acid derivatives with nanomolar agonist potencies at NMDA receptor subtypes (GluN12/A-D). These compounds are superagonists at GluN1/2C compared to glycine and partial to full agonists at GluN1/2A and GluN1/2D but display functional antagonism at GluN1/2B due to low agonist efficacy. Notably, 8d display 864% agonist efficacy at GluN1/2C relative to glycine, and 8j has high potency at GluN1/2A (0.018 μM), GluN1/2C (0.0029 μM), and GluN1/2D (0.016 μM). We evaluated the binding mode in the glycine site using molecular modeling and mutagenesis. In vitro absorption, distribution, metabolism, and excretion (ADME) assays predict high metabolic stability but poor blood–brain barrier permeability. However, an ester prodrug for the carboxylate group of 7j display moderately high blood–brain barrier permeability. The thiophenecarboxamide agonists expand the synthetic pharmacology of NMDA receptors and provide structural insights that facilitate the design of GluN1 agonists with GluN2 subunit-specific activity.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


