Isoelectric Point of Metal Oxide Films Formed by Anodization
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
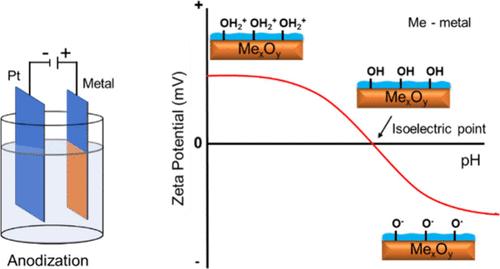
The surface charge of metal oxides is an important property that significantly contributes to a wide range of phenomena, including adsorption, catalysis, and material science. The surface charge can be predicted by determining the isoelectric point (IEP) of a material and the pH of a solution. Although there have been several studies of the IEP of metal oxide (nano)particles, only a few have reported the IEP of metal oxide films. The IEP of various compact metal oxide films such as TiO2, Nb2O5, WO3, ZrO2, NiO, and Al2O3 formed via electrochemical anodization was determined using the streaming potential technique. Nanostructured TiO2 and NiO were additionally produced using a single-step anodization technique, and their IEP was compared with the compact ones. The surface morphology and wettability of the oxides were studied by scanning electron microscopy and contact angle measurements, respectively. X-ray powder diffraction and X-ray photoelectron spectroscopy measurements were carried out to assess the phase and elemental composition, respectively. The IEP of compact anodic oxides deviates from that of their nanoparticle and atomic layer-deposited counterparts. The comparative results indicate that the IEP of metal oxides is influenced by factors such as the chemical composition, degree of hydroxylation, and crystallographic phases of the oxide.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


