Enhancing Photodynamic Therapy Efficacy via Photo-Triggered Calcium Overload and Oxygen Delivery in Tumor Hypoxia Management
IF 8.2
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Background: Photodynamic therapy (PDT) has emerged as a promising treatment for cancer, primarily due to its ability to generate reactive oxygen species (ROS) that directly induce tumor cell death. However, the hypoxic microenvironment commonly found within tumors poses a significant challenge by inhibiting ROS production. This study aims to investigate the effect of improving tumor hypoxia on enhancing PDT. Result: We employed polylactic-co-glycolic acid (PLGA) as a delivery vector for the encapsulation of indocyanine green (ICG), a photosensitizer, and perfluorohexane (PFH), with surface labeling mannose to facilitate targeted delivery. A potential therapeutic nanoplatform was fabricated, designated as Man-PFH-ICG@PLGA. These nanospheres are capable of localizing at tumor sites and can be tracked using photoacoustic (PA) imaging. Upon laser irradiation, the ROS generated by PDT activated the transient receptor potential cation channel subfamily A member 1 (TRPA1) located on the cell membrane. This activation led to an influx of extracellular Ca2+ and subsequently resulted in calcium overload. The excessive Ca2+ selectively accumulated in mitochondria, disrupting the function of enzymes involved in the mitochondrial respiratory chain. This disruption inhibits cellular respiration and decreases oxygen consumption in tumor cells, ultimately contributing to the alleviation of the hypoxic microenvironment within tumors. Simultaneously, PFH exhibited a high affinity for oxygen and can deliver exogenous oxygen directly to the tumor site through simple diffusion along the concentration gradient. Both the direct and indirect mechanisms synergistically contribute to ameliorating the hypoxic conditions within tumors, thereby augmenting the efficacy of PDT. Conclusions: The synergistic effect of photocontrolled calcium overload from endogenous sources and the oxygen-carrying nanoplatform alleviates tumor hypoxia, thereby enhancing the efficacy of PDT. This approach provides a new perspective on PDT.
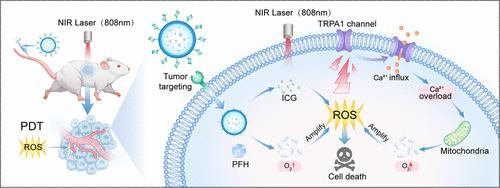
通过光触发钙超载和氧输送在肿瘤缺氧管理中增强光动力治疗效果
背景:光动力疗法(PDT)已经成为一种很有前景的癌症治疗方法,主要是因为它能够产生直接诱导肿瘤细胞死亡的活性氧(ROS)。然而,肿瘤内常见的低氧微环境通过抑制ROS的产生带来了重大挑战。本研究旨在探讨改善肿瘤缺氧对增强PDT的影响。结果:我们采用聚乳酸-羟基乙酸(PLGA)作为递送载体,对吲哚菁绿(ICG)、光敏剂和全氟己烷(PFH)进行了包封,表面标记甘露糖便于靶向递送。制备了一种潜在的治疗纳米平台,命名为Man-PFH-ICG@PLGA。这些纳米球能够定位在肿瘤部位,并可以使用光声成像(PA)进行跟踪。激光照射后,PDT产生的ROS激活了位于细胞膜上的瞬时受体电位阳离子通道亚家族A成员1 (TRPA1)。这种激活导致细胞外Ca2+的流入,随后导致钙超载。过量的Ca2+选择性地积聚在线粒体中,破坏了线粒体呼吸链中酶的功能。这种破坏抑制了细胞呼吸,减少了肿瘤细胞的耗氧量,最终有助于缓解肿瘤内的缺氧微环境。同时,PFH对氧表现出高亲和力,可以通过沿浓度梯度的简单扩散将外源氧直接输送到肿瘤部位。这两种直接和间接的机制协同作用有助于改善肿瘤内的缺氧条件,从而增强PDT的疗效。结论:内源光控钙超载与载氧纳米平台的协同作用可缓解肿瘤缺氧,从而增强PDT的疗效。这种方法为PDT研究提供了一个新的视角。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


