Insight into the Enhanced Removal of U(VI) with Fe–Ni Bimetallic Particles Loaded on Biochar
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
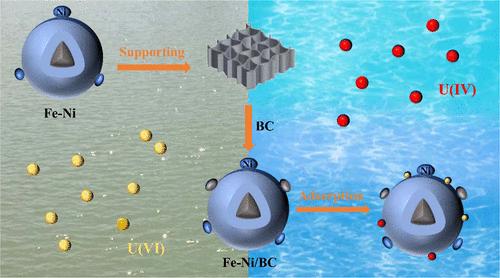
This work develops Fe–Ni particles loaded on biochar (Fe–Ni/BC) to remove U(VI) efficiently. Fe–Ni bimetallic particles loaded on biochar (BC) can improve stability and reactivity, and the mesoporous structure of BC can effectively reduce Fe0 aggregation. The removal ability of Fe–Ni/BC is higher than that of Fe–Ni, BC, and Fe/BC. With the aid of kinetics and isotherms, the removal data were fitted by the pseudo-second-order kinetic model (R2 ≥ 0.999) and Langmuir model (R2 ≥ 0.94). Meanwhile, Fe–Ni/BC exhibited the largest removal capacity of 250.78 mg/g for U(VI) at pH 5.0 and a temperature of 303 K. Removing uranium using Fe–Ni/BC was carried out in the following steps: First, U(VI) in the solution was sorbed onto the Fe–Ni/BC surface through chemical bonds. Second, Fe(II) and Fe0 contributed to the U(VI) reduction process. At the same time, Fe–Ni formed a primary cell and underwent electron transfer. Moreover, Ni0 adsorbed H2 generated by Fe0 corrosion, forming Ni–H to prevent agglomeration and reduce U(VI). The results indicate that Fe–Ni bimetallic particles loaded on biochar enhance the removal of U(VI) by sorption-reduction synergistic effect. This work offers valuable insights into the design of bimetallic nanomaterials for environmental remediation of U(VI) contamination.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Langmuir
化学-材料科学:综合
CiteScore
6.50
自引率
10.30%
发文量
1464
审稿时长
2.1 months
期刊介绍:
Langmuir is an interdisciplinary journal publishing articles in the following subject categories:
Colloids: surfactants and self-assembly, dispersions, emulsions, foams
Interfaces: adsorption, reactions, films, forces
Biological Interfaces: biocolloids, biomolecular and biomimetic materials
Materials: nano- and mesostructured materials, polymers, gels, liquid crystals
Electrochemistry: interfacial charge transfer, charge transport, electrocatalysis, electrokinetic phenomena, bioelectrochemistry
Devices and Applications: sensors, fluidics, patterning, catalysis, photonic crystals
However, when high-impact, original work is submitted that does not fit within the above categories, decisions to accept or decline such papers will be based on one criteria: What Would Irving Do?
Langmuir ranks #2 in citations out of 136 journals in the category of Physical Chemistry with 113,157 total citations. The journal received an Impact Factor of 4.384*.
This journal is also indexed in the categories of Materials Science (ranked #1) and Multidisciplinary Chemistry (ranked #5).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


