Extracellular barrier via in situ cross-linked catechol for blocking tumor mass transport and synergistic chemotherapy
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
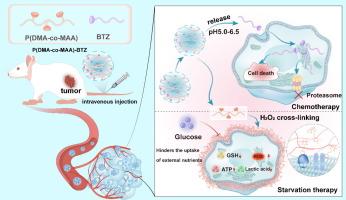
Due to the heightened nutritional consumption resulting from aberrant metabolism of tumor cells, starvation therapy, which involves blocking or depleting key nutrients in tumors, has gained popularity as an attractive approach to treating cancer. Herein, the catechol-containing polymer, P(DMA-co-MAA)-BTZ, was successfully synthesized via the copolymerizing dopamine methacrylamide (DMA) with polymethacrylic acid (MAA), followed by grafted with chemotherapy drug bortezomib (BTZ). The P(DMA-co-MAA)-BTZ exhibited pH-responsive behavior in the acidic tumor microenvironment, facilitating controlled release of BTZ for chemotherapy. Additionally, the liberated phenolic groups could form a membranal coating on the surface of tumor cells in response to overexpressed H2O2, thereby impeding the uptake of external nutrients and enabling starvation therapy. The efficacy of P(DMA-co-MAA)-BTZ in inhibiting glucose uptake by tumor cells has been demonstrated through both in vitro and in vivo experiments, resulting in a reduction of intracellular lactate, GSH, and ATP synthesis as well as the accumulation of reactive oxygen species as metabolic waste. This synergistic strategy of combining starvation therapy with chemotherapy yielded efficient anti-tumor effects while suppressing tumor metastasis and invasion, presenting a promising alternative approach for clinical tumor treatment.

求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


