Valorization of Hydrogen Peroxide for Sodium Percarbonate and Hydrogen Coproduction via Alkaline Water Electrolysis: Conceptual Process Design and Techno-Economic Evaluation
IF 3.8
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
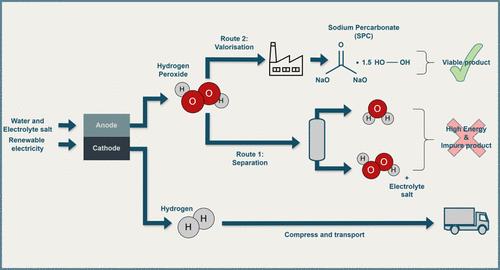
The recent interest in the production of green hydrogen through water electrolysis is hampered by its high cost when compared to steam methane reforming. To overcome this disadvantage, some studies explore replacing oxygen production with hydrogen peroxide at the anode, which has a higher value. Existing electrocatalysis research primarily focuses on hydrogen peroxide synthesis, neglecting process design and separation. Additionally, hydrogen peroxide’s thermodynamic instability in alkaline conditions and the existence of other ions make the separation difficult. This paper proposes a novel concept for the paired water electrolysis process that can be used to improve green hydrogen production economics through valuable chemical coproductions. Valorizing hydrogen peroxide to sodium percarbonate as the final product was chosen to address hydrogen peroxide separation challenges. An electrolyzer stack of 2 MW was chosen, incorporating a recirculating structure, and a boron-doped diamond anode to enhance the hydrogen peroxide production as the base case. According to the techno-economic analysis, for a 2 MW electrolyzer stack, capital expenditure was calculated as 64.5 M€, operational expenses as 21.6 M€, and revenue was calculated as 2.5 M€, resulting in a negative cash flow of −19.1 M€. Results revealed that the process can be profitable (breakeven point) at a capacity of approximately 308 electrolyzer stacks, which is 616 MW in capacity. A sensitivity analysis was conducted to determine how cost drivers including electricity price, anode price, Faradaic efficiency, price of the products and tax subsidy affect the breakeven point. A breakeven point of 60 electrolyzer stacks (120 MW) was found with a 100% increase in the sodium percarbonate sale price. In comparison, a theoretical 100% Faradaic efficiency in the anode material would result in a breakeven point of 38 electrolyzer stacks (76 MW). Even a more realistic 75% Faradaic efficiency leads to a breakeven plant size of 75 stacks (150 MW). Further, multiple two-parameter sensitivity analyses were conducted to assess the relations between Faradaic efficiency, sodium percarbonate sale price and anode material price. For instance, if sodium percarbonate price increases by 100% and Faradaic efficiency increases to 75%, the breakeven capacity drops down to 13 stacks (26 MW). Despite facing economic challenges for the proposed process design based on available technologies, the techno-economic analysis highlights key targets for future works. It also provides valuable insights into the economic feasibility of simultaneously producing hydrogen and sodium percarbonate through water electrolysis, indicating promising potential for the future.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


