Cobalt Nanoparticles Encapsulated in N-Doped Carbon Nanotubes Assembled on Carbon Cloth for Efficient Electroreduction of Nitrite to Ammonia
IF 3.9
3区 工程技术
Q2 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
Electrochemical nitrite (NO2–) reduction provides an alternative pathway for both sustainable ammonia (NH3) synthesis and reutilization of NO2– pollutants, but this process requires high activity and selective catalysts. In this work, cobalt nanoparticles encapsulated in N-doped carbon nanotubes supported on carbon cloth (Co@NCNT/CC) as a low-cost electrocatalyst can efficiently catalyze NO2–-to-NH3 conversion. Such Co@NCNT/CC shows exceptional electrocatalytic performance, achieving a maximum NH3 Faradaic efficiency of 94.9% with an NH3 yield of 365.1 μmol h–1 cm–2 at −0.3 V. Remarkably, the assembled Zn–NO2– battery with the Co@NCNT/CC cathode exhibits a peak power density of 4.4 mW cm–2 and a satisfactory NH3 yield of 141.5 μmol h–1 cm–2.
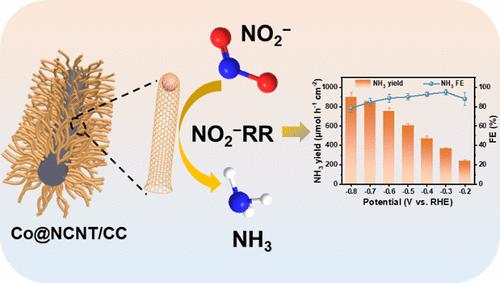
氮掺杂碳纳米管包裹的钴纳米颗粒在碳布上组装用于亚硝酸盐高效电还原制氨
电化学亚硝酸盐(NO2 -)还原为氨(NH3)的可持续合成和NO2 -污染物的再利用提供了另一种途径,但这一过程需要高活性和选择性的催化剂。在本研究中,钴纳米颗粒包裹在碳布(Co@NCNT/CC)上的n掺杂碳纳米管中,作为一种低成本的电催化剂,可以有效地催化NO2—到nh3的转化。Co@NCNT/CC表现出优异的电催化性能,在−0.3 V下NH3产率为365.1 μmol h-1 cm-2, NH3法拉第效率最高达94.9%。采用Co@NCNT/CC阴极制备的锌no2 -电池的峰值功率密度为4.4 mW cm-2, NH3产率为141.5 μmol h-1 cm-2,令人满意。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Industrial & Engineering Chemistry Research
工程技术-工程:化工
CiteScore
7.40
自引率
7.10%
发文量
1467
审稿时长
2.8 months
期刊介绍:
ndustrial & Engineering Chemistry, with variations in title and format, has been published since 1909 by the American Chemical Society. Industrial & Engineering Chemistry Research is a weekly publication that reports industrial and academic research in the broad fields of applied chemistry and chemical engineering with special focus on fundamentals, processes, and products.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


