Human subcutaneous and visceral adipocyte atlases uncover classical and nonclassical adipocytes and depot-specific patterns
IF 29
1区 生物学
Q1 GENETICS & HEREDITY
引用次数: 0
Abstract
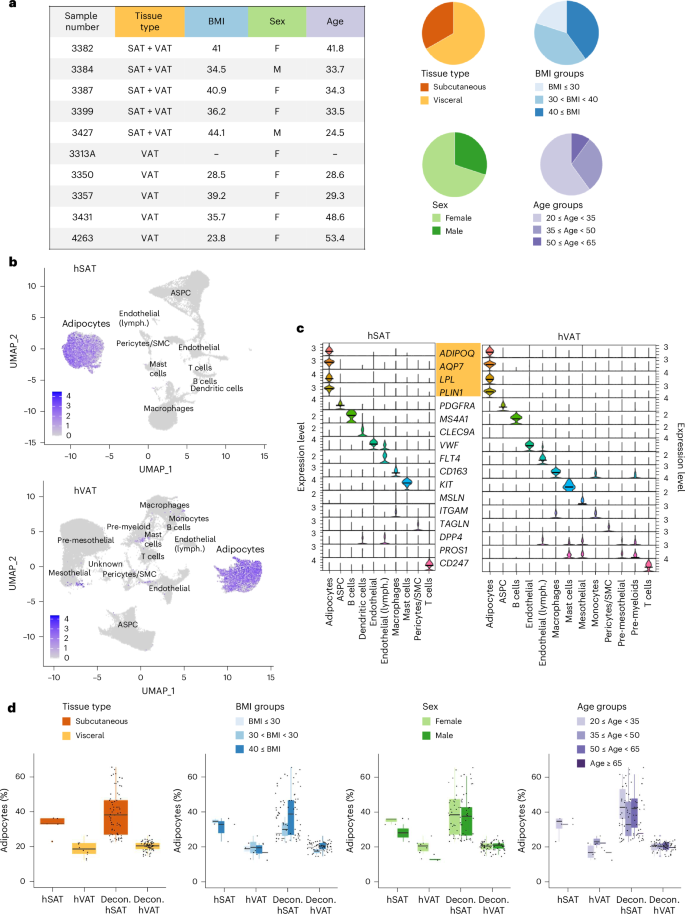
Human adipose depots are functionally distinct. Yet, recent single-nucleus RNA sequencing (snRNA-seq) analyses largely uncovered overlapping or similar cell-type landscapes. We hypothesized that adipocyte subtypes, differentiation trajectories and/or intercellular communication patterns could illuminate this depot similarity–difference gap. For this, we performed snRNA-seq of human subcutaneous or visceral adipose tissues (five or ten samples, respectively). Of 27,665 adipocyte nuclei in both depots, most were ‘classical’, namely enriched in lipid metabolism pathways. However, we also observed ‘nonclassical’ adipocyte subtypes, enriched in immune-related, extracellular matrix deposition (fibrosis), vascularization or angiogenesis or ribosomal and mitochondrial processes. Pseudo-temporal analysis showed a developmental trajectory from adipose progenitor cells to classical adipocytes via nonclassical adipocytes, suggesting that the classical state stems from loss, rather than gain, of specialized functions. Last, intercellular communication routes were consistent with the different inflammatory tone of the two depots. Jointly, these findings provide a high-resolution view into the contribution of cellular composition, differentiation and intercellular communication patterns to human fat depot differences. Single-nucleus RNA sequencing of human visceral and subcutaneous adipose tissues is used to identify adipocyte subpopulations and explore their developmental trajectories and interactions.

人皮下和内脏脂肪细胞图谱揭示经典和非经典脂肪细胞和仓库的特定模式
人类脂肪库在功能上是不同的。然而,最近的单核RNA测序(snRNA-seq)分析在很大程度上揭示了重叠或相似的细胞类型景观。我们假设脂肪细胞亚型、分化轨迹和/或细胞间通讯模式可以阐明这种相似性-差异性差距。为此,我们对人皮下或内脏脂肪组织(分别为5个或10个样本)进行了snrna测序。在两个储存库的27,665个脂肪细胞核中,大多数是“经典”的,即富含脂质代谢途径。然而,我们也观察到“非经典”脂肪细胞亚型,在免疫相关、细胞外基质沉积(纤维化)、血管形成或血管生成或核糖体和线粒体过程中富集。伪时间分析显示了由脂肪祖细胞经非经典脂肪细胞到经典脂肪细胞的发育轨迹,这表明经典状态源于特化功能的丧失,而不是获得。最后,细胞间通讯途径与两个库不同的炎症基调一致。总之,这些发现为细胞组成、分化和细胞间通讯模式对人类脂肪储存差异的贡献提供了高分辨率的视角。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature genetics
生物-遗传学
CiteScore
43.00
自引率
2.60%
发文量
241
审稿时长
3 months
期刊介绍:
Nature Genetics publishes the very highest quality research in genetics. It encompasses genetic and functional genomic studies on human and plant traits and on other model organisms. Current emphasis is on the genetic basis for common and complex diseases and on the functional mechanism, architecture and evolution of gene networks, studied by experimental perturbation.
Integrative genetic topics comprise, but are not limited to:
-Genes in the pathology of human disease
-Molecular analysis of simple and complex genetic traits
-Cancer genetics
-Agricultural genomics
-Developmental genetics
-Regulatory variation in gene expression
-Strategies and technologies for extracting function from genomic data
-Pharmacological genomics
-Genome evolution
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


