Nanoenzyme-Anchored Mitofactories Boost Mitochondrial Transplantation to Restore Locomotor Function after Paralysis Following Spinal Cord Injury
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Mitochondrial transplantation is a significant therapeutic approach for addressing mitochondrial dysfunction in patients with spinal cord injury (SCI), yet it is limited by rapid mitochondrial deactivation and low transfer efficiency. Here, high-quality mitochondria microfactories (HQ-Mitofactories) were constructed by anchoring Prussian blue nanoenzymes onto mesenchymal stem cells for effective mitochondrial transplantation to treat paralysis from SCI. Notably, the results demonstrated that HQ-Mitofactories could continuously produce vitality-boosting mitochondria with highly interconnected and elongated network structures under oxidative stress by scavenging excessive ROS. Furthermore, HQ-Mitofactories enabled efficient transfer of therapeutic mitochondria to injured neurons primarily via gap junctions, resulting in the restoration of mitochondrial homeostasis and thereby suppressing intracellular ROS burst and facilitating neuronal repair. After i.v. administration, HQ-Mitofactories migrated to the injured spinal cords of SCI mice and subsequently promoted neuronal regeneration and remyelination. Consequently, HQ-Mitofactory-treated mice successfully recovered locomotor function within 4 weeks, with 40% of the mice fully restoring walking after hindlimb paralysis. Conversely, untreated SCI exhibited completely abolished hindlimb movements. In light of real-time generation of vitality-boosting mitochondria even under oxidative stress and enabling targeted mitochondrial transfer, HQ-Mitofactories have promising therapeutic potential in the context of mitochondrial transplantation to reduce SCI-related paralysis, and more broadly impact the field of neuroregenerative medicine.
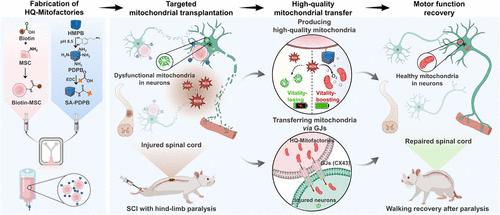
纳米酶锚定的有丝分裂工厂促进线粒体移植以恢复脊髓损伤后瘫痪的运动功能
线粒体移植是解决脊髓损伤(SCI)患者线粒体功能障碍的重要治疗方法,但线粒体失活快、转移效率低是其局限性。本研究通过将普鲁士蓝纳米酶锚定在间充质干细胞上,构建高质量的线粒体微工厂(HQ-Mitofactories),用于有效的线粒体移植治疗脊髓损伤性瘫痪。值得注意的是,研究结果表明,HQ-Mitofactories可以通过清除过量的ROS,在氧化应激下持续产生具有高度互联和细长网络结构的增强活力的线粒体。此外,HQ-Mitofactories使治疗性线粒体主要通过间隙连接有效地转移到受损神经元,从而恢复线粒体稳态,从而抑制细胞内ROS爆发,促进神经元修复。经静脉注射后,HQ-Mitofactories迁移到脊髓损伤小鼠,随后促进神经元再生和髓鞘再生。因此,hq - mitofactory治疗小鼠在4周内成功恢复运动功能,40%的小鼠在后肢瘫痪后完全恢复行走。相反,未经治疗的脊髓损伤表现出完全取消后肢运动。鉴于在氧化应激下也能实时生成增强活力的线粒体并实现靶向线粒体转移,HQ-Mitofactories在线粒体移植背景下具有良好的治疗潜力,以减少sci相关瘫痪,并更广泛地影响神经再生医学领域。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


