Multi-pathway and multiple-mechanism in action together: High-efficient visible-light photocatalytic oxidation and hydrolysis of CEES by a ligand-differentiated Zr-MOF
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
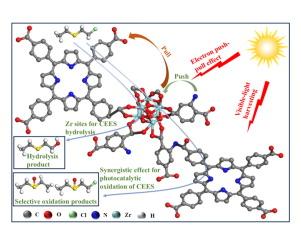
It is always important to develop materials that are capable of fast catalytically degrading sulfur mustard (HD) by selective oxidation and/or hydrolysis to its nontoxic form. In this paper, ligand-defected UiO-66-NH2 is firstly prepared using acetic acid as a coordinating modifier, and then 5,10,15,20-tetrakis(4-carboxyphenyl)porphyrin molecules are introduced by coordination and amidation reactions to form a new ligand-differentiated MOF UiO-66-NH-AA −TCPP. The new MOF achieves round-the-clock degradation of 2-chloroethyl ethyl sulfide (CEES, HD simulant) by visible-light photocatalytic oxidation and hydrolysis. Notably, the MOF degrades 97.8 % of CEES within 1 h with a half-life of 9.12 min via the cooperation of oxidation and hydrolysis. It is demonstrated that while TCPP increases the number of photoexcited electrons by enhancing visible-light absorption, the electron push–pull effect between –NH2 and –COOH groups of TCPP through the coordination reaction facilitates electron transfer along the ligand–metal cluster–ligand direction, which improves photocatalytic yield of 1O2 for selective oxidation of CEES to sulfoxide. Meanwhile, the MOF shows a stronger ability to hydrolyze CEES than UiO-66-NH2 due to the lipophilicity of porphyrin increasing the contact with CEES. Noteworthily, it is concluded that the MOF achieves efficient decontamination of CEES through a multi-pathway and multi-mechanism co-action strategy.
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


