Albumin-based delivery systems: Recent advances, challenges, and opportunities
IF 11.5
1区 医学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Albumin and albumin-based biomaterials have been explored for various applications, including therapeutic delivery, as therapeutic agents, as components of tissue adhesives, and in tissue engineering applications. Albumin has been approved as a nanoparticle containing paclitaxel (Abraxane®), as an albumin-binding peptide (Victoza®), and as a glutaraldehyde-crosslinked tissue adhesive (BioGlue®). Albumin is also approved as a supportive therapy for various conditions, including hypoalbuminemia, sepsis, and acute respiratory distress syndrome (ARDS). However, no other new albumin-based systems in a hydrogel format have been used in the clinic. A review of publicly available clinical trials indicates that no new albumin drug delivery formats are currently in the clinical development pipeline. Although albumin has shown promise as a carrier of therapeutics for various diseases, including diabetes, cancers, and infectious diseases, its potential for treating blood-borne diseases such as HIV and leukemia has not been translated. This review offers a perspective on the use of albumin-based drug delivery systems for a broader range of disease applications, considering the protein properties and a review of the currently approved albumin-based technologies. This review supports ongoing efforts to advance biomedical research and clinical interventions through albumin-based delivery systems.
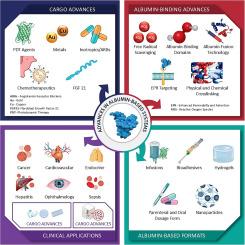
基于白蛋白的输送系统:最新进展、挑战和机遇。
白蛋白和基于白蛋白的生物材料已经探索了各种应用,包括治疗递送,作为治疗剂,作为组织粘接剂的成分,以及在组织工程中的应用。它已被批准作为含有紫杉醇(Abraxane®)的白蛋白纳米颗粒,作为白蛋白结合肽(Victoza®),以及作为戊二醛交联组织粘合剂(BioGlue®)。白蛋白也被批准作为多种疾病的支持治疗,包括低白蛋白血症、败血症和急性呼吸窘迫综合征(ARDS)。然而,没有其他新的基于白蛋白的水凝胶系统在临床中使用。对公开临床试验的回顾表明,目前没有新的白蛋白药物递送形式处于临床开发管道中。尽管白蛋白已显示出作为多种疾病(包括糖尿病、癌症和传染病)的治疗载体的希望,但其治疗血液传播疾病(如艾滋病毒和白血病)的潜力尚未得到证实。本文综述了基于白蛋白的药物传递系统在更广泛的疾病应用中的应用,考虑了蛋白质的特性,并综述了目前批准的基于白蛋白的技术。本综述支持通过基于白蛋白的输送系统推进生物医学研究和临床干预的持续努力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Controlled Release
医学-化学综合
CiteScore
18.50
自引率
5.60%
发文量
700
审稿时长
39 days
期刊介绍:
The Journal of Controlled Release (JCR) proudly serves as the Official Journal of the Controlled Release Society and the Japan Society of Drug Delivery System.
Dedicated to the broad field of delivery science and technology, JCR publishes high-quality research articles covering drug delivery systems and all facets of formulations. This includes the physicochemical and biological properties of drugs, design and characterization of dosage forms, release mechanisms, in vivo testing, and formulation research and development across pharmaceutical, diagnostic, agricultural, environmental, cosmetic, and food industries.
Priority is given to manuscripts that contribute to the fundamental understanding of principles or demonstrate the advantages of novel technologies in terms of safety and efficacy over current clinical standards. JCR strives to be a leading platform for advancements in delivery science and technology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


