Honeysuckle-Derived Carbon Dots With Robust Catalytic and Pharmacological Activities for Mitigating Lung Inflammation by Inhibition of Caspase11/GSDMD-Dependent Pyroptosis
IF 18.5
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The catalytic activity of carbon dots (CDs) has generated significant interest regarding their potential applications within the biomedical field. However, the structure-activity relationship of CDs and their pharmacological mechanisms in disease treatment have yet to be comprehensively elucidated. In this study, two distinct types of CDs exhibiting superoxide dismutase (SOD)-like enzymatic activities are synthesized through hydrothermal (Hy-CDs) and carbonization (Ca-CDs) methods, utilizing Honeysuckle as the common carbon material precursor. Through comparative analysis, surface group modifications, and theoretical calculations, it is determined that the SOD-like enzymatic activity of CDs primarily originated from the stabilizing influence of the amino group on the superoxide (•O2−) intermediate and its conjugation to the π-system, facilitating electron transfer. In vitro experiments demonstrated that Hy-CDs effectively alleviated cellular oxidative stress and inhibited the secretion of pro-inflammatory cytokines. Furthermore, the significant bioactivity and catalytic properties of Hy-CDs contribute to their pronounced therapeutic efficacy in the treatment of acute lung injury (ALI) and lung ischemia/reperfusion injury (LIRI). Guided by transcriptomic analysis and Western blotting, it is demonstrated that Hy-CDs effectively inhibit Caspase11/GSDMD-dependent non-classical pyroptosis by down-regulating GBP2 protein expression, thereby contributing to lung inflammation. This study elucidates the structure-activity relationship and underlying biological mechanisms of Hy-CDs in therapeutic applications.
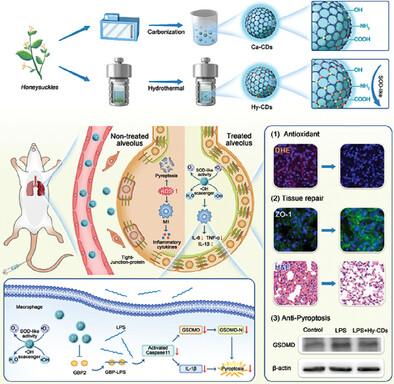
金银花衍生碳点通过抑制Caspase11/ gsdmd依赖性热凋亡,具有强大的催化和药理活性,可减轻肺部炎症
碳点(cd)的催化活性引起了人们对其在生物医学领域潜在应用的极大兴趣。然而,CDs的构效关系及其在疾病治疗中的药理机制尚未得到全面阐明。本研究利用金银花作为常见碳材料前体,通过水热法(hyp -CDs)和碳化法(Ca-CDs)合成了两种不同类型的具有超氧化物歧化酶(SOD)样酶活性的CDs。通过比较分析、表面基团修饰和理论计算,确定CDs类sod酶活性主要源于氨基对超氧化物(•O2−)中间体的稳定作用及其与π体系的共轭作用,促进了电子转移。体外实验表明,Hy-CDs能有效缓解细胞氧化应激,抑制促炎细胞因子的分泌。此外,Hy-CDs显著的生物活性和催化特性使其在治疗急性肺损伤(ALI)和肺缺血再灌注损伤(LIRI)方面具有显著的疗效。在转录组学分析和Western blotting的指导下,我们发现Hy-CDs通过下调GBP2蛋白表达,有效抑制Caspase11/ gsdmd依赖性非经典焦亡,从而导致肺部炎症。本研究阐明了Hy-CDs在治疗应用中的构效关系及其潜在的生物学机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


