Base-Promoted [4 + 1 + 1] Multicomponent Tandem Cycloaddition of Ortho-Substituted Nitroarenes, Aldehydes, and Ammonium Salts To Access 2,4-Substituted Quinazoline Frameworks
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
We report a base-promoted, metal-free multicomponent tandem reaction, involving a [4 + 1 + 1] cycloaddition process between ortho-substituted nitroarenes, aldehydes, and ammonium salts. Modifying the substituents on the nitroaromatic compounds effectively provides structurally diverse 2-substituted and 4-alkenylquinazolines with good to excellent yields (77%–90% and quinazoline 51 examples) and high tolerance for various inorganic ammonium salts (13 examples, such as NH3·H2O, NH4Cl, and NH4HF2). A new method for constructing 2,4-substituted quinazoline compounds with high selectivity from simple nitrogen source compounds was developed, and the reaction can be scaled up to a gram scale. Additionally, this method also facilitates the preparation of organic molecules with photophysical properties, offering new insights into the further transformation of quinazolines.
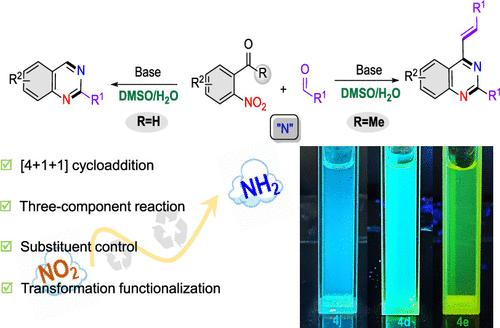
邻位取代硝基芳烃、醛和铵盐的碱促进[4 + 1 + 1]多组分串联环加成制得2,4-取代喹唑啉骨架
我们报道了一个碱促进的,无金属的多组分串联反应,涉及邻取代硝基芳烃,醛和铵盐之间的[4 + 1 + 1]环加成过程。在硝基芳香族化合物上进行取代基修饰,可有效地获得结构多样的2-取代基和4-烯基喹唑啉类化合物,产率优良(77% ~ 90%,喹唑啉51例),对多种无机铵盐(NH3·H2O、NH4Cl、NH4HF2等13例)具有较高的耐受性。提出了一种以简单氮源化合物为原料合成高选择性2,4-取代喹唑啉化合物的新方法,该方法可将反应规模扩大到克级。此外,该方法还有助于制备具有光物理性质的有机分子,为喹唑啉类化合物的进一步转化提供了新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


