Butyl Imidates: Highly Stable and Isolable Synthetic Intermediates
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Imidates are versatile synthetic intermediates that contain ambiphilic reactivity, making them valuable pharmaceutically relevant synthons. Despite their extensive utility, imidates are typically generated in situ rather than isolated due to their inherent instability. This report details a systematic study that led to the discovery of an isolable imidate hydrogen chloride (HCl) salt that exhibits high tolerance to hydrolysis, thereby improving process control and facilitating downstream transformations. Optimization of reaction conditions and anti-solvent selection resulted in a general and scalable approach to access the imidate HCl salt in high yield. A multikilogram campaign of the butyl imidate demonstrated key improvements over the ethyl congener, addressing key practical challenges such as solubility, hydrolysis, and impurity formation. The scope was extended to a series of phenyl- and benzyl-substituted n-butyl imidates.
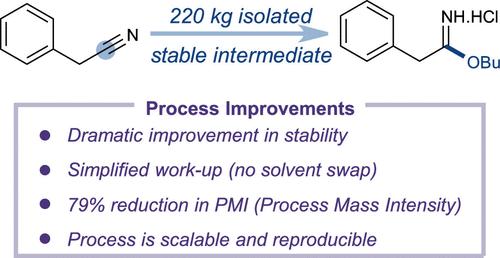
丁基酸酯:高度稳定和可分离的合成中间体
邻苯二甲酸酯是一种多用途的合成中间体,具有两亲性反应性,使其具有重要的药学意义。尽管具有广泛的用途,但由于其固有的不稳定性,它们通常是就地生成的,而不是分离出来的。本报告详细介绍了一项系统研究,该研究发现了一种可分离的酰亚胺氯化氢(HCl)盐,该盐具有很高的水解耐受性,从而改善了工艺控制并促进了下游转化。通过对反应条件的优化和抗溶剂的选择,获得了一种通用的、可扩展的高产亚咪酯HCl盐制备方法。一项多千克的运动证明了与乙基同系物相比,亚酰丁酯的关键改进,解决了诸如溶解度、水解和杂质形成等关键的实际挑战。范围扩展到一系列苯基和苄基取代的正丁基近似物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


