Impact and removal of fluorine impurity in the comprehensive recovery of spent LiFePO4/C
IF 8.1
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
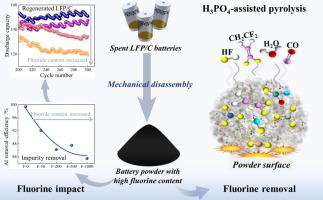
The comprehensive recovery of spent lithium iron phosphate powder (LFP/C) remains challenging in industry due to the difficulty in impurity removal. Specifically, the impact of fluorine impurity on the recovery process is unclear. In this work, the specific effects of fluorine on the removal of aluminum impurities and the subsequent recovery of FePO4·2H2O from spent LFP/C were investigated, and an acid-assisted pyrolysis process was proposed to transfer fluorine species into the gas phase for fluorine removal. The results indicate that due to the coordination reactions between F- with Al3+ and Fe2+/Fe3+, the presence of F- in increased the difficulty of aluminum removal and reduced the precipitation efficiency of FePO4·2H2O. Additionally, F- accelerated the aging of LFP/C cathode materials, increasing resistance to lithium-ion migration, which ultimately resulted in an irreversible decline in electrochemical performance. The acid-assisted pyrolysis process achieved a fluorine removal rate of approximately 98 % under the optimal condition (pyrolysis temperature 600-700°C, reaction time 4.0h, H3PO4 dosage 1.2 times of theoretic amount, and solid/liquid ratio 4.0), reducing the fluorine content from 1.69 wt% to 0.05 wt%. This work presents a potential strategy for fluorine removal, contributing to the comprehensive recovery of valuable elements from spent LFP/C.
废LiFePO4/C综合回收中氟杂质的影响及去除
废磷酸铁锂粉(LFP/C)的综合回收由于杂质去除困难,在工业上一直是一个挑战。具体来说,氟杂质对回收过程的影响尚不清楚。本文研究了氟对铝杂质的去除和废LFP/C中FePO4·2H2O的回收的具体影响,并提出了一种酸助热解工艺,将氟转移到气相中进行除氟。结果表明:由于F-与Al3+和Fe2+/Fe3+的配位反应,F- in的存在增加了除铝的难度,降低了FePO4·2H2O的沉淀效率;此外,F-加速了LFP/C正极材料的老化,增加了对锂离子迁移的抵抗力,最终导致电化学性能不可逆转地下降。在最佳条件下(热解温度600 ~ 700℃,反应时间4.0h, H3PO4投加量为理论投加量的1.2倍,料液比4.0),酸助热解工艺的氟去除率约为98 %,氟含量由1.69 wt%降至0.05 wt%。这项工作提出了一种潜在的除氟战略,有助于从废LFP/C中全面回收有价值元素。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Separation and Purification Technology
工程技术-工程:化工
CiteScore
14.00
自引率
12.80%
发文量
2347
审稿时长
43 days
期刊介绍:
Separation and Purification Technology is a premier journal committed to sharing innovative methods for separation and purification in chemical and environmental engineering, encompassing both homogeneous solutions and heterogeneous mixtures. Our scope includes the separation and/or purification of liquids, vapors, and gases, as well as carbon capture and separation techniques. However, it's important to note that methods solely intended for analytical purposes are not within the scope of the journal. Additionally, disciplines such as soil science, polymer science, and metallurgy fall outside the purview of Separation and Purification Technology. Join us in advancing the field of separation and purification methods for sustainable solutions in chemical and environmental engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


