Rationally designed highly amphipathic antimicrobial peptides demonstrating superior bacterial selectivity relative to the corresponding α-helix peptide
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
De novo design of antimicrobial peptides is a pivotal strategy for developing new antibacterial agents, leveraging its rapid and efficient nature. (XXYY)n, where X represents cationic residues, Y denotes hydrophobic residues, and n varies from 2 to 4, is a classical α-helix template. Based on which, numerous antimicrobial peptides have been synthesized. Herein, we hypothesize that the amphipathy of this type of α-helix template can be further enhanced based on the principles of α-helical protein folding, characterized by a rotation occurring every 3.6 amino acid residues, and propose the highly amphipathic template XXYYXXYXXYYX (where X represents cationic residues and Y denotes hydrophobic residues). Accordingly, the amino acid composition and arrangement of the α-helix peptide (RRWF)3 are adjusted, yielding the highly amphipathic counterpart H–R (RRWFRRWRRWFR). The structure-activity relationship of which is further explored through the substitution of residues at positions 8 and 12. Notably, the highly amphipathic peptides exhibit enhanced antimicrobial activity and reduced hemolytic toxicity compared to (RRWF)3, resulting in superior bacterial selectivity. The most highly amphipathic peptide, H–R, demonstrates potent activity against biofilms and multidrug-resistant bacteria, low propensity for resistance, and high safety and effectiveness in vivo. The antibacterial mechanisms of H–R are also preliminarily investigated in this study. As noted, H–R represents a promising antimicrobial candidate for addressing infections associated with drug-resistant bacteria.

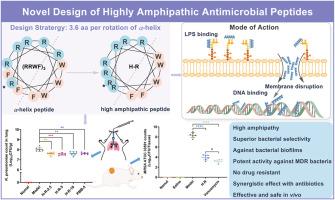
合理设计的高度两亲抗菌肽相对于相应的α-螺旋肽具有优越的细菌选择性
抗菌肽的从头设计是开发新型抗菌药物的关键策略,利用其快速和高效的性质。(XXYY)n,其中X为阳离子残基,Y为疏水性残基,n取值范围为2 ~ 4,是经典的α-螺旋模板。在此基础上,合成了许多抗菌肽。本文基于α-螺旋蛋白每3.6个氨基酸残基旋转一次的折叠原理,假设该型α-螺旋模板的两亲性可以进一步增强,并提出高度两亲性模板XXYYXXYXXYYX(其中X代表阳离子残基,Y代表疏水残基)。相应地,α-螺旋肽(RRWF)3的氨基酸组成和排列进行了调整,生成了高度两亲性的H-R (RRWFRRWRRWFR)。通过取代第8位和第12位的残基,进一步探讨了其构效关系。值得注意的是,与(RRWF)3相比,高度两亲肽表现出增强的抗菌活性和降低的溶血毒性,从而产生优越的细菌选择性。两亲性最强的肽H-R对生物膜和多重耐药细菌具有较强的活性,耐药倾向低,在体内安全性和有效性高。本研究还对H-R的抗菌机制进行了初步探讨。如上所述,H-R代表了解决与耐药细菌相关的感染的有希望的抗微生物候选药物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


