Closing the Loop on Lithium-Ion Battery Cathodes: A Green Electrometallurgical Recycling Approach
IF 7.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The burgeoning use of lithium-ion batteries (LIBs) creates a growing challenge: spent battery management. Traditional hydrometallurgical recycling with coprecipitation generates massive Na2SO4 wastewater, posing a significant environmental burden. This work presents a novel, closed-loop recycling method for LIB cathode materials that merges electrolysis and hydrometallurgy. Using Na2SO4 electrolysis, we produced high-purity sulfuric acid and sodium hydroxide solutions, which served as the key reagents for leaching and resynthesizing waste cathodes. Optimized leaching conditions ensure near-complete recovery of valuable metals. Li2CO3 and a precursor (Ni0.8Co0.1Mn0.1(OH)2) are subsequently precipitated and regenerated into a new LiNi0.8Co0.1Mn0.1O2 cathode material, which demonstrates excellent electrochemical performance. The spent Na2SO4 solution undergoes a simple treatment before re-electrolysis, achieving a closed-loop system with minimal waste generation and reduced reliance on external reagents. Moreover, the acid-leaching carbon residue is repurposed as a bifunctional carbon-based catalyst for hydrogen peroxide production. This innovative approach offers both economic and environmental benefits, paving the way for sustainable LIBs recycling and a circular economy for battery materials.
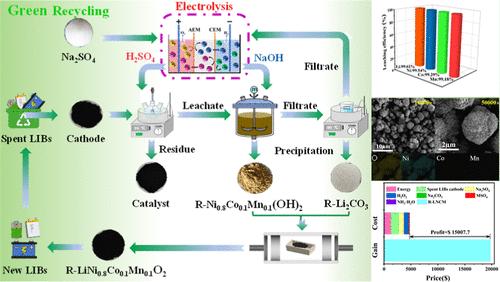
锂离子电池阴极闭合回路:一种绿色电冶金回收方法
锂离子电池(lib)的迅速使用带来了一个日益严峻的挑战:废旧电池的管理。传统的共沉淀法湿法冶金回收产生了大量的Na2SO4废水,造成了严重的环境负担。本研究提出了一种新颖的锂离子电池正极材料闭环回收方法,该方法将电解和湿法冶金相结合。采用Na2SO4电解法制备高纯硫酸和氢氧化钠溶液,作为废阴极浸出和再合成的关键试剂。优化的浸出条件可确保几乎完全回收有价金属。Li2CO3与前驱体Ni0.8Co0.1Mn0.1(OH)2析出再生为新的LiNi0.8Co0.1Mn0.1O2正极材料,具有优异的电化学性能。废Na2SO4溶液在再电解前经过简单处理,实现了一个闭环系统,产生的废物最少,减少了对外部试剂的依赖。此外,酸浸碳渣被重新利用为双氧水生产的双功能碳基催化剂。这种创新的方法提供了经济和环境效益,为可持续的锂离子电池回收和电池材料的循环经济铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


