Switchable Hydrophilicity Amine Product Extraction: Efficient Separation of Tertiary Amines via Carbon Dioxide-Induced Polarity Switch in Homogeneous Catalysis
IF 7.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
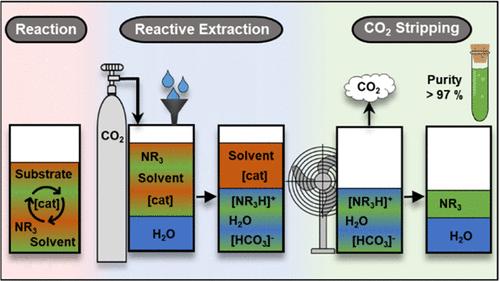
A combined approach is presented for the homogeneously catalyzed synthesis of tertiary amines and their subsequent carbon dioxide (CO2)-induced separation. After production, the nonpolar tertiary amines are reacted with carbon dioxide to the corresponding polar ammonium carbonates and separated from the nonpolar reaction phase by water extraction. Subsequently, the nonpolar tertiary amines reform upon CO2-stripping the loaded aqueous phase, enabling their straightforward isolation by decantation. For this Switchable Hydrophilicity Amine Product Extraction (SHAPE) concept, the influence of various nonpolar solvents on the separation efficiency was investigated using N,N-dimethylbenzylamine (DMBA) as the model amine. After examination of all operating parameters of SHAPE in anisole as the best-performing solvent, homogeneously Ru-catalyzed alcohol amination of benzyl alcohol was identified as the most suitable model reaction for the production of DMBA, which was successfully isolated in 84.9% yield using SHAPE methodology. Moreover, only 0.09 wt % of Ru was lost in the separated product phase. This SHAPE concept was successfully combined with catalysis in thermomorphic multiphase systems (TMS). Four different tertiary amines were efficiently separated from the nonpolar solvent and the other reaction components, achieving high product purities of up to 97.3 wt %.
可切换亲水性胺产物萃取:二氧化碳诱导极性开关在均相催化下高效分离叔胺
提出了一种用于均相催化合成叔胺及其随后的二氧化碳诱导分离的组合方法。生产后,非极性叔胺与二氧化碳反应生成相应的极性碳酸铵,用水萃取分离出非极性反应相。随后,非极性叔胺在二氧化碳剥离负载的水相上重整,使其能够通过滗析直接分离。以N,N-二甲基苄胺(DMBA)为模型胺,研究了不同非极性溶剂对可切换亲水性胺产物萃取(SHAPE)效率的影响。在以苯甲醚为最佳溶剂的条件下,考察了SHAPE的所有操作参数,确定了均相钌催化苯甲醇醇胺化反应是最适合生产DMBA的模型反应,并利用SHAPE方法成功分离出DMBA,收率为84.9%。分离产物相中Ru的损失仅为0.09 wt %。该SHAPE概念成功地与热致多相系统(TMS)的催化相结合。四种不同的叔胺从非极性溶剂和其他反应组分中有效分离,产品纯度高达97.3%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


