Intensification of Renewable 4,4′-Dimethylbiphenyl Synthesis for Recyclable Diesters
IF 7.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Reducing the global dependence on petroleum-derived chemical products requires renewable alternatives to replace established materials. Recent investigations demonstrated a biobased pathway to prepare the platform chemical 4,4′-dimethylbiphenyl (4,4′-DMBP). The synthesis of 4,4′-DMBP follows a two-step process: (1) 2-methylfuran (2-MF) oxidative coupling to 5,5′-dimethyl-2,2′-bifuran (5,5′-DMBF) and (2) 5,5′-DMBF tandem Diels–Alder-dehydration with ethylene to afford 4,4′-DMBP. Here, we report the intensification of reaction conditions in step (1), improving 5,5′-DMBF space-time yield up to 1.10 mol L–1h–1, an 86% increase from the baseline. Scale-up of step (1) was hindered by oxygen-deprivation-induced palladium black formation and reaction exotherms decreasing yields at larger scales. Oxygen sparging, mechanical mixing, and internal cooling implemented simultaneously enabled a 108× increase in 5,5′-DMBF production to an average of 13 g/batch. In step (2), the use of a homogeneous La(OTf)3 catalyst in the Diels–Alder-dehydration reaction─instead of heterogeneous γ-Al2O3─led to a 54% increase in 4,4′-DMBP yield with a 70 °C temperature reduction to 180 °C. Scale-up of the Diels–Alder-dehydration to 3 g/batch maintained para-selectivity for 4,4′-DMBP with full conversion to the product within 20 h. Renewable 4,4′-DMBP is achieved from the improved pathway and isolated in 96.7% purity for further utilization downstream.
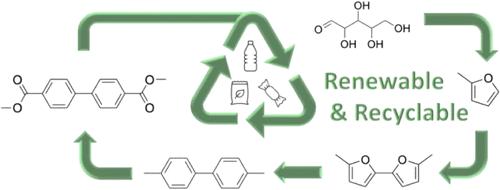
可再生4,4′-二甲基联苯酯合成的强化研究
减少全球对石油衍生化学产品的依赖需要可再生替代品来取代现有材料。最近的研究证明了一种生物基途径可以制备平台化学品4,4 ' -二甲基联苯(4,4 ' -DMBP)。4,4 ' -DMBP的合成遵循两个步骤:(1)2-甲基呋喃(2- mf)氧化偶联到5,5 ' -二甲基-2,2 ' -双脲(5,5 ' -DMBF)和(2)5,5 ' -DMBF串联diels - alder与乙烯脱水得到4,4 ' -DMBP。在这里,我们报告了步骤(1)中反应条件的强化,将5,5 ' -DMBF时空产率提高到1.10 mol L-1h-1,比基线提高了86%。步骤(1)的扩大受到缺氧诱导的钯黑形成和反应放热在更大规模上降低产率的阻碍。氧气喷射、机械混合和内部冷却同时实施,使5.5 ' -DMBF产量增加108倍,平均每批产量达到13克。在步骤(2)中,在diels - alder -脱水反应中使用均相的La(OTf)3催化剂(而不是非均相的γ-Al2O3),导致4,4 ' -DMBP产率提高54%,温度降低70℃至180℃。将diols - alder脱水放大至3g /批,保持了4,4 ' -DMBP的准选择性,并在20小时内完全转化为产品。改进的途径可再生4,4 ' -DMBP,纯度为96.7%,可进一步用于下游利用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


