In2O3 modification mitigates Jahn-Teller effect in LiMn2O4 enhancing lithium extraction efficiency and stability
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
The rapid growth of lithium-ion batteries has intensified the demand for lithium, requiring the development of efficient selective extraction methods from salt-lake brines with high magnesia-lithium ratios. This work presents a novel composite electrode material, In2O3-modified LiMn2O4 (In-LMO), synthesized through solvent evaporation coupled with the calcination method, for Selective electro-chemical extraction of lithium. The introduction of In2O3 effectively modulates the local charge state within the LMO structure, resulting in an increased proportion of MnVI and mitigating the detrimental Jahn-Teller effect, which typically compromises stability during lithium extraction. Characterizations confirm that the anchoring of In2O3 also enhances the stability of lattice oxygen in LMO. The optimized 1In-LMO electrode material demonstrates impressive lithium extraction capacities of 20.18 mg/g in simulated brine, with minimal Mn dissolution and excellent cycling stability. Notably, in the mother liquor of Li2CO3 with a high Na/Li ratio (44.8), the system achieves a selective lithium release capacity of 21.33 mg/g, while in the challenging West Taijinar salt lake brine with elevated Mg/Li ratios, a selective extraction capacity of 26.75 mg/g was attained. These results underscore the industrial potential of the 1In-LMO electrode material, positioning it as a promising candidate for sustainable lithium recovery from complex brine sources.
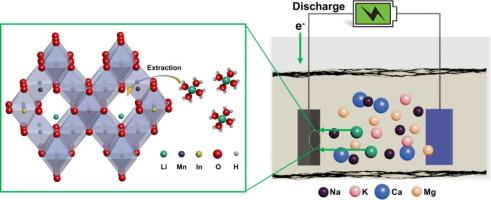
In2O3改性降低了LiMn2O4中的Jahn-Teller效应,提高了锂萃取效率和稳定性
锂离子电池的快速发展加剧了对锂的需求,需要开发高效的选择性提取方法,从高镁锂比的盐湖盐水中提取锂。采用溶剂蒸发与煅烧相结合的方法合成了一种新型的复合电极材料——in2o3修饰的LiMn2O4 (In-LMO),用于锂的选择性电化学萃取。In2O3的引入有效地调节了LMO结构内的局部电荷状态,导致MnVI的比例增加,并减轻了有害的Jahn-Teller效应,该效应通常会影响锂提取过程的稳定性。表征证实了In2O3的锚定也增强了LMO中晶格氧的稳定性。优化后的1In-LMO电极材料在模拟盐水中的锂萃取量为20.18 mg/g, Mn溶解最小,循环稳定性好。值得注意的是,在高Na/Li比(44.8)的Li2CO3母液中,该体系的锂选择性释放量为21.33 mg/g,而在高mg/ Li比的西台湾盐湖卤水中,该体系的锂选择性提取量为26.75 mg/g。这些结果强调了1In-LMO电极材料的工业潜力,将其定位为从复杂卤水来源中可持续回收锂的有前途的候选材料。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


