Cobalt-doping mediated low-valence Rh centers in rhodium sulfide superconductor for improved electrocatalytic hydrogen evolution
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Doping is an effective strategy to boost the hydrogen evolution reaction (HER) performance. Here, we construct a Co-doping Rh17S15 electrocatalyst fabricated using a green method, avoiding the use of any organic solvent. These fabricated electrocatalysts exhibit superior HER activity both in acidic and alkaline media. In 0.5 M H2SO4 electrolyte, Rh16.85Co0.15S15 has an apparent overpotential and Tafel slope decrease of 135.8 mV and 46.2 mV dec-1 compared with Rh17S15. Besides, the electrochemical active surface area (ECSA) of Rh16.85Co0.15S15 is around 5.0 times that of Rh17S15. While in 1 M KOH electrolyte, the Rh16.85Co0.15S15 also exhibits the most outstanding HER activity with an evident overpotential, Tafel slope, and Rct declining of 78.9 mV, 78.4 mV dec-1 and 13.6 Ω compared with Rh17S15. Moreover, the ECSA of Rh16.85Co0.15S15 is 1.7 times that of Rh17S15. Although the low values are a little far from the commercial Pt/C (20%) of 46.8 mV in an acidic medium, these low values are close to the commercial Pt/C (20%) of 62.8 mV in an alkaline medium. First-principles calculations show that Co-doping decreases the reaction energy barrier and thus improves the electrocatalysis performance of Co-doping Rh17S15. Beyond offering an advanced electrocatalyst in different media, this work guides the designing and tuning properties of electrocatalysts.
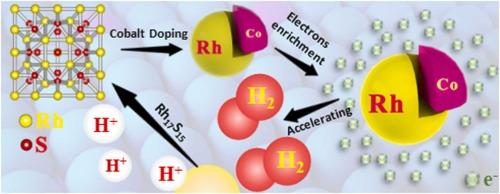
钴掺杂在硫化铑超导体中介导的低价Rh中心用于改进电催化析氢
掺杂是提高析氢反应(HER)性能的有效策略。在这里,我们构建了一个共掺杂的Rh17S15电催化剂,采用绿色方法制备,避免使用任何有机溶剂。这些制备的电催化剂在酸性和碱性介质中均表现出优异的HER活性。在0.5 M H2SO4电解液中,与Rh17S15相比,Rh16.85Co0.15S15的过电位和Tafel斜率分别降低了135.8 mV和46.2 mV dec1。此外,Rh16.85Co0.15S15的电化学活性表面积(ECSA)是Rh17S15的5.0倍左右。而在1 M KOH电解液中,Rh16.85Co0.15S15的HER活性也最为突出,与Rh17S15相比,其过电位、Tafel斜率明显,Rct分别下降78.9 mV、78.4 mV dec1和13.6 Ω。此外,Rh16.85Co0.15S15的ECSA是Rh17S15的1.7倍。虽然在酸性介质中,这些低值与46.8 mV的商业Pt/C(20%)相差甚远,但在碱性介质中,这些低值接近62.8 mV的商业Pt/C(20%)。第一性原理计算表明,共掺杂降低了反应能垒,从而提高了共掺杂Rh17S15的电催化性能。除了在不同介质中提供先进的电催化剂外,这项工作还指导了电催化剂的设计和调谐性能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Alloys and Compounds
工程技术-材料科学:综合
CiteScore
11.10
自引率
14.50%
发文量
5146
审稿时长
67 days
期刊介绍:
The Journal of Alloys and Compounds is intended to serve as an international medium for the publication of work on solid materials comprising compounds as well as alloys. Its great strength lies in the diversity of discipline which it encompasses, drawing together results from materials science, solid-state chemistry and physics.
文献相关原料
公司名称
产品信息
麦克林
cobalt powder
阿拉丁
sulfur powder
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


