Optimizing Ni MEPCM catalysts for thermal regulation in CO2 methanation: ZrO2-CaO surface doping
IF 13.3
1区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
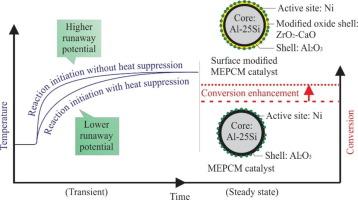
CO2 methanation has become a crucial part of the carbon recycling process owing to its potential to reduce greenhouse gas emissions from renewable hydrogen sources. The reaction is highly exothermic and, under inadequate control, may cause thermal runaway and lead to catalyst deactivation. Therefore, thermal regulation is crucial for ensuring optimum productivity. Microencapsulated phase change materials (MEPCM) were introduced as a promising thermal regulation approach because of their superior capability in controlling the system temperature using the latent heat storage (LHS) concept. This study aims to improve the effectiveness of MEPCM as catalysts support by incorporating mass transfer agent for CO2 methanation process, building upon prior research which focuses on direct impregnation on MEPCM support. To accomplish this goal, a novel MEPCM catalyst structure was developed by loading a Ni metal active site onto the ZrO2-CaO surface-enhanced MEPCM. Novel MEPCM catalysts were prepared using three sequential methods: MEPCM synthesis, surface modification, and catalyst impregnation. Characterization analysis revealed that the modification improved the surface area by 45%. ZrO2-CaO doping successfully enhanced the catalytic performance by increasing the CO2 conversion and CH4 selectivity by 8 and 6%, respectively. In addition, the LHS function of the catalyst-loaded MEPCM suppressed the rapid increase in temperature during the initiation of CO2 methanation. Further development of this concept is expected to enhance the efficiency of runway reaction control across numerous chemical industries.

优化Ni MEPCM催化剂用于CO2甲烷化的热调节:ZrO2-CaO表面掺杂
二氧化碳甲烷化已成为碳回收过程的关键部分,因为它有可能减少可再生氢源的温室气体排放。该反应是高度放热的,如果控制不当,可能导致热失控并导致催化剂失活。因此,热调节是确保最佳生产力的关键。微封装相变材料(MEPCM)是一种很有前途的热调节方法,因为它具有利用潜热存储(LHS)概念控制系统温度的优越能力。本研究旨在在前人直接浸渍MEPCM载体的基础上,通过加入传质剂提高MEPCM作为CO2甲烷化催化剂载体的有效性。为了实现这一目标,通过在ZrO2-CaO表面增强MEPCM上加载Ni金属活性位点,开发了一种新的MEPCM催化剂结构。采用MEPCM合成、表面改性和催化剂浸渍三个步骤制备了新型MEPCM催化剂。表征分析表明,改性后的表面面积提高了45%。ZrO2-CaO掺杂成功地提高了催化剂的CO2转化率和CH4选择性,分别提高了8%和6%。此外,负载催化剂的MEPCM的LHS功能抑制了CO2甲烷化起始过程中温度的快速升高。这一概念的进一步发展有望提高众多化工行业跑道反应控制的效率。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemical Engineering Journal
工程技术-工程:化工
CiteScore
21.70
自引率
9.30%
发文量
6781
审稿时长
2.4 months
期刊介绍:
The Chemical Engineering Journal is an international research journal that invites contributions of original and novel fundamental research. It aims to provide an international platform for presenting original fundamental research, interpretative reviews, and discussions on new developments in chemical engineering. The journal welcomes papers that describe novel theory and its practical application, as well as those that demonstrate the transfer of techniques from other disciplines. It also welcomes reports on carefully conducted experimental work that is soundly interpreted. The main focus of the journal is on original and rigorous research results that have broad significance. The Catalysis section within the Chemical Engineering Journal focuses specifically on Experimental and Theoretical studies in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. These studies have industrial impact on various sectors such as chemicals, energy, materials, foods, healthcare, and environmental protection.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


