Engineered Extracellular Vesicles from Antler Blastema Progenitor Cells: A Therapeutic Choice for Spinal Cord Injury
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
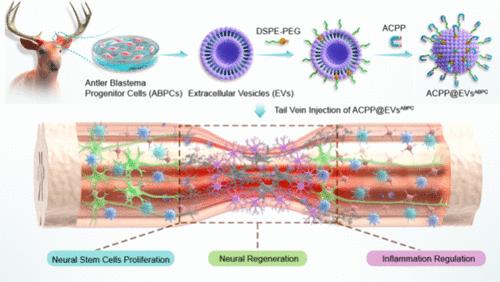
Deer antler blastema progenitor cells (ABPCs) are promising for regenerative medicine due to their role in annual antler regeneration, the only case of complete organ regeneration in mammals. ABPC-derived signals show great potential for promoting regeneration in tissues with limited natural regenerative ability. Our findings demonstrate the capability of extracellular vesicles from ABPCs (EVsABPC) to repair spinal cord injury (SCI), a condition with low regenerative capacity. EVsABPC significantly enhanced the proliferation of neural stem cells (NSCs) and activated neuronal regenerative potential, resulting in a 5.2-fold increase in axonal length. Additionally, EVsABPC exhibited immunomodulatory effects, shifting macrophages from M1 to M2. Engineered with activated cell-penetrating peptides (ACPPs), EVsABPC significantly outperformed EVs from rat bone marrow stem cells (EVsBMSC) and neural stem cells (EVsNSC), promoting a 1.3-fold increase in axonal growth, a 30.6% reduction in neuronal apoptosis, and a 2.6-fold improvement in motor function recovery. These findings support ABPC-derived EVs as a promising therapeutic candidate for SCI repair.
鹿角胚祖细胞的工程细胞外囊泡:脊髓损伤的治疗选择
鹿角胚基祖细胞(ABPCs)是哺乳动物中唯一完全器官再生的动物,它在鹿角的年度再生中发挥着重要的作用,在再生医学中具有广阔的应用前景。在自然再生能力有限的组织中,abpc来源的信号显示出促进再生的巨大潜力。我们的研究结果表明,ABPCs细胞外囊泡(EVsABPC)具有修复脊髓损伤(SCI)的能力,这是一种再生能力较低的疾病。EVsABPC显著增强神经干细胞(NSCs)的增殖,激活神经元再生潜能,导致轴突长度增加5.2倍。此外,EVsABPC表现出免疫调节作用,将巨噬细胞从M1转移到M2。利用活化的细胞穿透肽(ACPPs), EVsABPC显著优于来自大鼠骨髓干细胞(EVsBMSC)和神经干细胞(EVsNSC)的EVs,促进轴突生长增加1.3倍,神经元凋亡减少30.6%,运动功能恢复改善2.6倍。这些发现支持abpc衍生的ev作为一种有希望的脊髓损伤修复治疗候选者。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


