EXOTLR1/2-STING: A Dual-Mechanism Stimulator of Interferon Genes Activator for Cancer Immunotherapy
IF 16
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
As natural agonists of the stimulator of interferon genes (STING) protein, cyclic dinucleotides (CDNs) can activate the STING pathway, leading to the expression of type I interferons and various cytokines. Efficient activation of the STING pathway in antigen-presenting cells (APCs) and tumor cells is crucial for antitumor immune response. Tumor-derived exosomes can be effectively internalized by APCs and tumor cells and have excellent potential to deliver CDNs to the cytoplasm of APCs and tumor cells. Here, we leverage tumor exosomes as a delivery platform, designing an EXOTLR1/2-STING loaded with CDNs. To achieve efficient loading of CDNs onto exosomes, we chemically conjugated CDNs with Pam3CSK4, a compound featuring multiple fatty acid chains, resulting in Pam3CSK4–CDGSF. Utilizing the high lipophilicity of Pam3CSK4, Pam3CSK4–CDGSF could be efficiently loaded onto the exosomes through simple incubation. Moreover, as an agonist for Toll-like receptor 1/2, Pam3CSK4 also exhibits robust immunological synergistic effects in conjunction with CDNs. EXOTLR1/2-STING effectively induced the activation of APCs and triggered tumor cell death, producing a favorable antitumor therapeutic effect. It also demonstrated significant synergistic effects with immune checkpoint therapies.
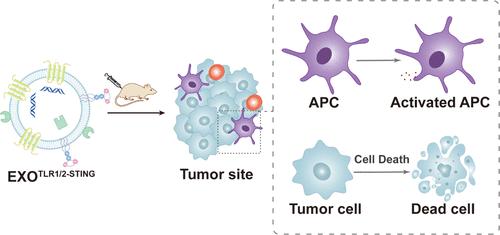
EXOTLR1/2-STING:肿瘤免疫治疗干扰素基因激活剂的双机制刺激剂
环二核苷酸(cyclic dinucleotides, cdn)作为干扰素基因刺激因子(STING)蛋白的天然激动剂,可以激活STING通路,导致I型干扰素和各种细胞因子的表达。抗原呈递细胞(APCs)和肿瘤细胞中STING通路的有效激活对于抗肿瘤免疫应答至关重要。肿瘤源性外泌体可被apc和肿瘤细胞有效内化,具有向apc和肿瘤细胞的细胞质输送cdn的良好潜力。在这里,我们利用肿瘤外泌体作为递送平台,设计了装载cdn的EXOTLR1/2-STING。为了有效地将cdn装载到外体上,我们将cdn与具有多个脂肪酸链的化合物Pam3CSK4化学偶联,得到Pam3CSK4 - cdgsf。利用Pam3CSK4的高亲脂性,Pam3CSK4 - cdgsf可以通过简单的孵育有效地装载到外泌体上。此外,作为toll样受体1/2的激动剂,Pam3CSK4还与cdn一起表现出强大的免疫协同作用。EXOTLR1/2-STING可有效诱导apc活化,触发肿瘤细胞死亡,具有良好的抗肿瘤治疗效果。它还显示出与免疫检查点疗法显著的协同作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


