Ethane Oxidative Dehydrogenation over TiO2 and M/TiO2 Catalysts: Unraveling the Surface Structure Evolution, Oxygen Species Type, and Role of Doped Metal in Tuning Catalytic Performance
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
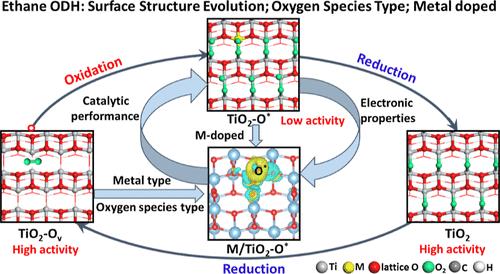
TiO2 has better catalytic performance toward alkane oxidative dehydrogenation (ODH) due to adjustable surface oxygen species; however, identifying the dynamic evolution process of the TiO2 surface structure and its effect on the type of surface oxygen species is still challenging. In this work, the combined methods of density functional theory calculations and kinetic Monte Carlo simulations were employed to fully investigate the catalytic performance of ethane ODH over TiO2 and 15 types of single-atom metal-doped TiO2 (M/TiO2) catalysts. The results clearly unravel the evolution mechanism of the TiO2 surface structure and the type of surface oxygen species formed during the evolution process in tuning ethane ODH catalytic performance. Surface oxygen vacancies enhance catalytic performance with unsaturated Ti4CN as the active site, while surface-adsorbed oxygen species limit catalytic performance. Single-atom metal-doped TiO2 can change the O2(g) adsorption mode and dissociation activity to adjust the type of surface oxygen species and further regulate the catalytic performance by tuning electronic properties of adsorbed oxygen atoms. Interestingly, the screened V/TiO2–O* catalyst exhibits high C2H4(g) production activity and selectivity at the optimal temperature of 873.15 K and a C2H6(g) partial pressure of 0.2 bar, which thoroughly eliminates the negative effect of adsorbed oxygen species over the TiO2 catalyst in the process of ethane ODH due to more charge transfer from V to the adsorbed oxygen atom. This work provides the theoretical basis and structural clue for designing an alkane ODH catalyst by regulating the types and electronic properties of surface oxygen species over metal oxide.
TiO2和M/TiO2催化剂上乙烷氧化脱氢:揭示表面结构演变、氧种类类型和掺杂金属在调节催化性能中的作用
TiO2由于表面氧种类可调节,对烷烃氧化脱氢(ODH)具有较好的催化性能;然而,确定TiO2表面结构的动态演变过程及其对表面氧种类的影响仍然是一个挑战。本文采用密度泛函理论计算和动力学蒙特卡罗模拟相结合的方法,全面研究了乙烷ODH对TiO2和15种单原子金属掺杂TiO2 (M/TiO2)催化剂的催化性能。研究结果清楚地揭示了TiO2表面结构的演化机制以及在调整乙烷ODH催化性能的演化过程中形成的表面氧的种类。以不饱和Ti4CN为活性位点的表面氧空位提高了催化性能,而表面吸附的氧限制了催化性能。单原子金属掺杂TiO2可以通过改变O2(g)的吸附方式和解离活性来调节表面氧的种类,并通过调节被吸附氧原子的电子性质来进一步调节催化性能。有趣的是,所筛选的V/TiO2 - o *催化剂在最佳温度873.15 K和C2H6(g)分压0.2 bar时表现出较高的C2H4(g)生成活性和选择性,彻底消除了乙烷ODH过程中吸附氧对TiO2催化剂的负面影响,因为V向吸附氧原子转移了更多的电荷。本研究为通过调节金属氧化物表面氧的种类和电子性质来设计烷烃ODH催化剂提供了理论依据和结构线索。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


