A Pseudo-Block Copolymerization Access to Cyclic Alternating Copolymers through Segment-Selective Transesterification
IF 5.1
Q1 POLYMER SCIENCE
引用次数: 0
Abstract
Efficient synthesis of cyclic polymers remains a frontier challenge. We report here that macromolecular transesterification during a pseudoblock copolymerization process can be utilized for such a purpose. Organobase-catalyzed ring-opening alternating copolymerization of 3,4-dihydrocoumarin and epoxide is conducted with four-armed poly(ethylene oxide) (PEO) as a macroinitiator. Intramolecular transesterification (backbiting) occurs selectively on the newly formed polyester segments. The disconnected cyclic alternating copolymers can be easily isolated by precipitation owing to their substantial solubility difference from the PEO-containing acyclic parts. The obtained cyclic alternating copolymers exhibit low dispersity (<1.2) and a molar mass of around 3 kg mol–1, irrespective of the monomer-to-initiator feed ratio, indicating thermodynamic control over the ring size. The macrocyclic structure is confirmed by both mass spectroscopy and microscopic visualization and then utilized to prepare cyclic-brush terpolymer by thiol–ene modification, followed by graft polymerization of propylene oxide.
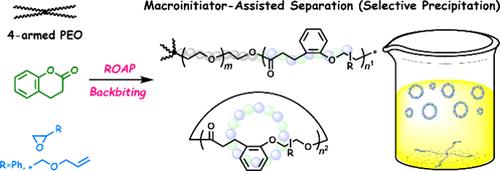
通过段选择性酯交换反应获得环交替共聚物的伪嵌段共聚
高效合成环状聚合物仍然是一个前沿挑战。我们在这里报告,在伪嵌段共聚过程中的大分子酯交换可以用于这样的目的。以四臂聚环氧乙烷(PEO)为宏观引发剂,进行了有机碱催化3,4-二氢香豆素与环氧化物开环交替共聚反应。分子内酯交换反应选择性地发生在新形成的聚酯片段上。由于其溶解度与含peo的无环部分存在很大差异,因此可以很容易地通过沉淀分离出断开的环状交替共聚物。所得到的环状交替共聚物具有低分散性(<1.2)和约3kg mol-1的摩尔质量,与单体与引发剂的投料比无关,表明热力学对环尺寸的控制。通过质谱分析和微观可视化验证了其大环结构,并利用巯基改性法制备了环刷型三元共聚物,再进行环氧丙烷接枝聚合。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.40
自引率
3.40%
发文量
209
审稿时长
1 months
期刊介绍:
ACS Macro Letters publishes research in all areas of contemporary soft matter science in which macromolecules play a key role, including nanotechnology, self-assembly, supramolecular chemistry, biomaterials, energy generation and storage, and renewable/sustainable materials. Submissions to ACS Macro Letters should justify clearly the rapid disclosure of the key elements of the study. The scope of the journal includes high-impact research of broad interest in all areas of polymer science and engineering, including cross-disciplinary research that interfaces with polymer science.
With the launch of ACS Macro Letters, all Communications that were formerly published in Macromolecules and Biomacromolecules will be published as Letters in ACS Macro Letters.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


