Engineering Cellular Vesicles for Immunotherapy
IF 14
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
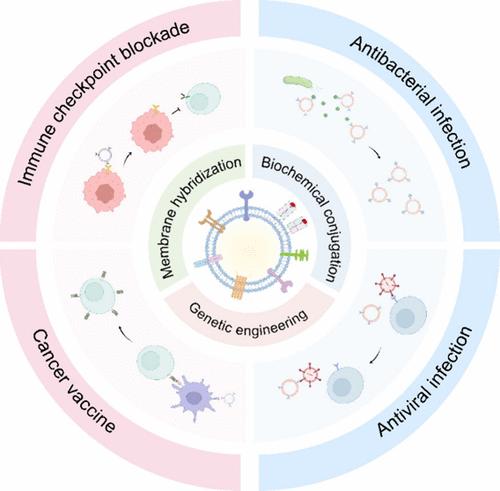
Immunotherapy has become a crucial strategy for cancer and infectious diseases due to its ability to leverage the power of the immune system to combat diseases, particularly when conventional therapeutic options have been ineffective. Nonetheless, low immune response rates and immune-related adverse events (irAEs) remain significant challenges for immunotherapeutics. Therefore, there is an urgent need to develop new strategies for improving the immunotherapy. Extracellular vesicles (EVs), secreted by living cells, are small membrane-bound vesicles. Their size varies from 30 to 150 nm in diameter and can be found in various bodily fluids, including blood, tears, and breast milk. They have attracted extensive attention in immunotherapy due to their integral role in essential physiological and pathological processes. Despite their potential, EVs face limitations, including low productivity and high costs, hindering their clinical applications. These issues have recently been addressed with the advent of EV mimics. EV mimics are artificially produced nanoscale vesicles. Compared to EVs, they offer superior production efficiency while maintaining similar biological properties. EV mimics are obtained by physical methods from natural cells. Methods such as serial extrusion, sonication, and electroporation are now used to produce synthetic EV mimics, making them viable for immunotherapy applications. Building on this, we have developed various EV mimics from different cell sources for immunotherapy and engineering natural EVs and EV mimics using chemical and bioengineering strategies like biochemical conjugation, genetic engineering, and membrane hybridization. These engineered natural EVs and EV mimics have controllable immunomodulatory properties, capable of modulating (i.e., boosting or inhibiting) immunity for the treatment of cancer and infectious diseases.
工程细胞囊泡免疫治疗
免疫疗法已成为癌症和传染病的关键策略,因为它能够利用免疫系统的力量来对抗疾病,特别是在传统治疗方案无效的情况下。然而,低免疫应答率和免疫相关不良事件(irAEs)仍然是免疫疗法面临的重大挑战。因此,迫切需要制定新的策略来改善免疫治疗。细胞外囊泡(EVs)是由活细胞分泌的小的膜结合囊泡。它们的直径从30纳米到150纳米不等,可以在各种体液中发现,包括血液、眼泪和母乳。由于它们在重要的生理和病理过程中起着不可或缺的作用,在免疫治疗中引起了广泛的关注。尽管潜力巨大,但电动汽车仍面临生产力低、成本高等限制,阻碍了其临床应用。随着电动汽车的出现,这些问题最近得到了解决。EV模拟物是人工制造的纳米级囊泡。与电动汽车相比,它们在保持类似生物特性的同时,提供了更高的生产效率。EV模拟物是通过物理方法从自然细胞中获得的。诸如连续挤压,超声和电穿孔等方法现在用于生产合成的EV模拟物,使其适用于免疫治疗应用。在此基础上,我们利用化学和生物工程策略,如生化偶联、基因工程和膜杂交,从不同的细胞来源开发了各种EV模拟物,用于免疫治疗和工程天然EV和EV模拟物。这些工程化的天然EV和EV模拟物具有可控的免疫调节特性,能够调节(即增强或抑制)治疗癌症和传染病的免疫。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


