Confining Surface Oxygen Redox in Double Perovskites for Enhanced Oxygen Evolution Reaction Activity and Stability
IF 24.4
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Nickel-based double perovskites AA′BB′O6 are an underexplored class of oxygen evolution reaction (OER) catalysts, in which B-site substitution is used to tune electronic and structural properties. BaSrNiWO6, with a B-site comprised of alternating Ni and W, exhibits high oxygen evolution activity, attributed to the evolution of a highly OER active surface phase. The redox transformation of Ni2+(3d8) to Ni3+(3d7) combined with partial W dissolution into the electrolyte from the linear Ni(3d)-O(2p)-W(5d) chains drives an in situ reconstruction of the surface to an amorphized, NiO-like layer, promoting oxygen redox in the OER mechanism. However, the high valence W6+(5d0) acts as a stabilizing electronic influence in the bulk, preventing the mobilization of lattice oxygen which is bound in highly covalent W─O bonds. It is proposed that the surface generated during the OER can support a lattice oxygen evolution mechanism (LOEM) in which oxygen vacancies are created and preferentially refilled by electrolytic OH−, while bulk O species remain stable. This surface LOEM (sLOEM) allows BaSrNiWO6 to retain structural integrity during OER catalysis. With a Tafel slope of 45 mV dec−1 in 0.1 m KOH, BaSrNiWO6 illustrates the potential of Ni-based double perovskites to offer both OER efficiency and bulk stability in alkaline electrolysis.
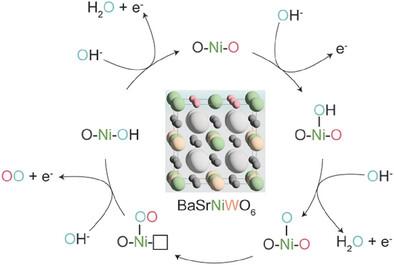
限制双钙钛矿表面氧氧化还原以增强析氧反应活性和稳定性
镍基双钙钛矿AA 'BB 'O6是一类尚未开发的析氧反应(OER)催化剂,其中使用b位取代来调整电子和结构性质。b位由Ni和W交替组成的BaSrNiWO6表现出高的析氧活性,这是由于高OER活性表面相的演化。Ni2+(3d8)氧化还原转化为Ni3+(3d7),并结合线性Ni(3d)-O(2p)-W(5d)链部分W溶解到电解质中,驱动表面原位重建为非晶化的nio样层,促进OER机制中的氧氧化还原。然而,高价价W6+(5d0)在体中起着稳定电子影响的作用,阻止了以高共价W─O键结合的晶格氧的动员。在OER过程中产生的表面可以支持晶格析氧机制(LOEM),在该机制中,氧空位产生并优先被电解OH -重新填充,而大块O保持稳定。这种表面LOEM (sLOEM)允许BaSrNiWO6在OER催化过程中保持结构完整性。在0.1 m KOH条件下,BaSrNiWO6的Tafel斜率为45 mV dec−1,表明了镍基双钙钛矿在碱性电解中提供OER效率和体积稳定性的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Energy Materials
CHEMISTRY, PHYSICAL-ENERGY & FUELS
CiteScore
41.90
自引率
4.00%
发文量
889
审稿时长
1.4 months
期刊介绍:
Established in 2011, Advanced Energy Materials is an international, interdisciplinary, English-language journal that focuses on materials used in energy harvesting, conversion, and storage. It is regarded as a top-quality journal alongside Advanced Materials, Advanced Functional Materials, and Small.
With a 2022 Impact Factor of 27.8, Advanced Energy Materials is considered a prime source for the best energy-related research. The journal covers a wide range of topics in energy-related research, including organic and inorganic photovoltaics, batteries and supercapacitors, fuel cells, hydrogen generation and storage, thermoelectrics, water splitting and photocatalysis, solar fuels and thermosolar power, magnetocalorics, and piezoelectronics.
The readership of Advanced Energy Materials includes materials scientists, chemists, physicists, and engineers in both academia and industry. The journal is indexed in various databases and collections, such as Advanced Technologies & Aerospace Database, FIZ Karlsruhe, INSPEC (IET), Science Citation Index Expanded, Technology Collection, and Web of Science, among others.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


