Radially Distributed Electron Transfer on Single-Crystalline Surface of Gold Microplates
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Electron transfer is ubiquitous in many chemical reactions and biological phenomena; however, the spatial heterogeneities of electron transfer kinetics in electrocatalysis are so far insufficiently resolved. Measuring and understanding the localized electron transfer are crucial to deciphering the intrinsic activity of electrocatalysts and to achieving further improvements in performance. By using scanning electrochemical probe microscopy to spatially resolve redox electrochemistry across the single-crystalline surface of gold microplates, we discover an intriguing radially distributed electron transfer pattern, where the kinetics around the periphery region are significantly higher than those at the central region, regardless of the redox reaction types. In combination with atomic force microscopy-based infrared spectroscopy for synergistic interrogation of local chemical heterogeneities, we deduce that such a radial pattern of electron transfer originates from the uneven distribution of passive adlayer across the microplate surface. Subsequently, we verify that the spatial heterogeneity of electron transfer can be eliminated by removing the surface adlayer by either mild room temperature aging or oxygen plasma exposure. In addition to gaining insight into the spatial heterogeneities of electron transfer at the nanoscale, our work highlights the important effect of adsorbed organic species at nanocrystal surfaces on electrocatalysis.
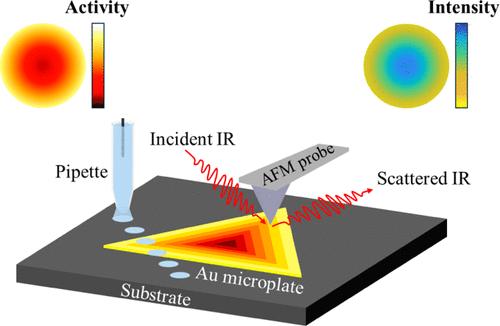
金微孔板单晶表面径向分布的电子转移
电子转移在许多化学反应和生物现象中是普遍存在的;然而,电催化过程中电子传递动力学的空间非均质性尚未得到充分解决。测量和理解局域电子转移对于破译电催化剂的内在活性和实现性能的进一步改进至关重要。通过使用扫描电化学探针显微镜对金微孔板单晶表面的氧化还原电化学进行空间解析,我们发现了一个有趣的径向分布的电子转移模式,无论氧化还原反应类型如何,周围区域的动力学都明显高于中心区域的动力学。结合基于原子力显微镜的红外光谱对局部化学异质性的协同询问,我们推断这种电子转移的径向模式源于微孔板表面被动层的不均匀分布。随后,我们验证了通过温和的室温老化或氧等离子体暴露去除表面涂层可以消除电子转移的空间异质性。除了深入了解纳米尺度下电子转移的空间异质性外,我们的工作还强调了纳米晶体表面吸附的有机物质对电催化的重要影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


