H4K12 lactylation-regulated NLRP3 is involved in cigarette smoke-accelerated Alzheimer-like pathology through mTOR-regulated autophagy and activation of microglia
IF 12.2
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
Cigarette smoke (CS), an indoor environmental pollution, is an environmental risk factor for diverse neurological disorders. However, the neurotoxicological effects and mechanisms of CS on Alzheimer's disease (AD) progression remain unclear. We found that CS accelerated the progression of AD, including increasing β-amyloid (Aβ) plaque deposition and exacerbating cognitive decline. Mechanistically, CS exposure increased the levels of NOD-like receptor protein 3 (NLRP3), which impaired autophagic flux in microglia by activating the mammalian target of rapamycin (mTOR) signal. Metabolomics analysis revealed an upregulation of lactate levels and an increase in global protein lysine lactylation in the brain tissue of CS-exposed AD-transgenic mice. Immunoprecipitation-Mass Spectrometry and chromatin immunoprecipitation assays demonstrated that CS elevates H4K12 lactylation (H4K12la) levels, which accumulate at the promoter region of NLRP3, leading to the activation of its transcription. Via inhibiting lactate or NLRP3 activation, oxamate and MCC950 alleviates these CS-induced effects. Therefore, our data suggest that the CS-induced increase in lactate levels triggers NLRP3 transcriptional activation through H4K12la, which subsequently leads to mTOR-mediated autophagy dysfunction in microglia, promoting microglial activation and resulting in Aβ plaque accumulation in AD-transgenic mice. This provides a new mechanism and potential therapeutic target for AD associated with environmental factors.
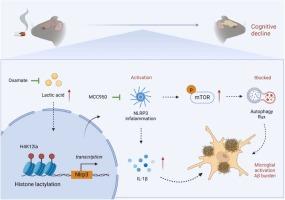
H4K12乳酸化调节的NLRP3通过mtor调节的自噬和小胶质细胞的激活参与香烟烟雾加速的阿尔茨海默病样病理
香烟烟雾是一种室内环境污染,是多种神经系统疾病的环境危险因素。然而,CS对阿尔茨海默病(AD)进展的神经毒理学作用和机制尚不清楚。我们发现CS加速了AD的进展,包括增加β-淀粉样蛋白(Aβ)斑块沉积和加剧认知能力下降。从机制上讲,CS暴露增加了nod样受体蛋白3 (NLRP3)的水平,通过激活哺乳动物雷帕霉素靶蛋白(mTOR)信号来破坏小胶质细胞的自噬通量。代谢组学分析显示,cs暴露的ad转基因小鼠脑组织中乳酸水平上调,整体蛋白赖氨酸乳酸化增加。免疫沉淀-质谱和染色质免疫沉淀试验表明,CS提高了H4K12乳酸化(H4K12la)水平,这些水平积聚在NLRP3的启动子区域,导致其转录激活。通过抑制乳酸或NLRP3的激活,草酸酯和MCC950减轻了cs诱导的这些效应。因此,我们的数据表明,cs诱导的乳酸水平升高通过H4K12la触发NLRP3转录激活,随后导致mtor介导的小胶质细胞自噬功能障碍,促进小胶质细胞活化,导致ad转基因小鼠Aβ斑块积累。这为与环境因素相关的AD提供了新的机制和潜在的治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Hazardous Materials
工程技术-工程:环境
CiteScore
25.40
自引率
5.90%
发文量
3059
审稿时长
58 days
期刊介绍:
The Journal of Hazardous Materials serves as a global platform for promoting cutting-edge research in the field of Environmental Science and Engineering. Our publication features a wide range of articles, including full-length research papers, review articles, and perspectives, with the aim of enhancing our understanding of the dangers and risks associated with various materials concerning public health and the environment. It is important to note that the term "environmental contaminants" refers specifically to substances that pose hazardous effects through contamination, while excluding those that do not have such impacts on the environment or human health. Moreover, we emphasize the distinction between wastes and hazardous materials in order to provide further clarity on the scope of the journal. We have a keen interest in exploring specific compounds and microbial agents that have adverse effects on the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


