In Situ Slow-Release Hydrogen Sulfide Therapeutics for Advanced Disease Treatments
IF 13
2区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
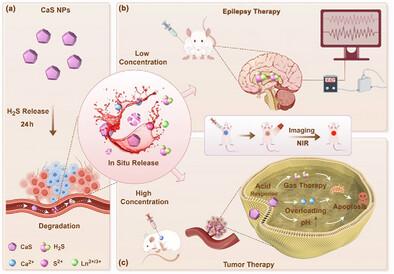
Hydrogen sulfide (H2S) gas therapygarners significant attention for its potential to improve outcomes in various disease treatments. The quantitative control of H2S release is crucial for effective the rapeutic interventions; however, traditional researchon H2S therapy frequently utilizes static release models and neglects the dynamic nature of blood flow. In this study, we propose a novel slow-release in-situ H2S release model that leverages the dynamic hydrolysis of H2S donorswithin the bloodstream. Calcium sulfide nanoparticles (CaS NPs) withmicrosolubility characteristics exhibit continuous H2S release, surpassing 24 h at normal blood flow velocities. The extended-release profile demonstrates superior potential in aligning with the bell-shapedpharmacological effect of H2S, compared to NaHS. Moreover, we synthesisedrare earth-doped CaS NPs (CaS: Eu2+, Sm3+ NPs) tha texhibit persistent luminescence, enabling visualisation of the continuous H2S release in trials. Our results demonstrate that lowdose CaS: Eu2+, Sm3+ NPs significantly reduces seizureduration to 1.2 ± 0.7 minutes, while high dose effectively suppresses colontumor growth with a tumor inhibition rate of 54%. Remarkably, these findings closely resemble endogenous H2S levels in treating epilepsy and tumors. This innovative slow-release, in-situ H2S the rapeutic approach via hydrolysis rejuvenates the development of H2S-basedtherapeutics.
原位缓释硫化氢治疗晚期疾病
硫化氢(H2S)气体疗法因其改善各种疾病治疗结果的潜力而备受关注。H2S释放的定量控制是有效治疗干预的关键;然而,传统的H2S治疗研究往往采用静态释放模型,忽视了血流的动态性。在这项研究中,我们提出了一种新的H2S原位缓释模型,该模型利用了血液中H2S供体的动态水解。具有微溶解度特性的硫化钙纳米颗粒(CaS NPs)在正常血流速度下可连续释放H2S超过24小时。与NaHS相比,H2S的缓释谱显示出与钟形药理效应一致的优越潜力。此外,我们合成了稀土掺杂的CaS NPs (CaS: Eu2+, Sm3+ NPs),这些NPs表现出持续发光,可以在试验中看到连续的H2S释放。我们的研究结果表明,低剂量的CaS: Eu2+, Sm3+ NPs显著减少了1.2±0.7分钟的癫痫发作,而高剂量的CaS: Eu2+, Sm3+ NPs有效抑制了结肠瘤的生长,肿瘤抑制率为54%。值得注意的是,这些发现与治疗癫痫和肿瘤的内源性H2S水平非常相似。这种创新的通过水解的原位缓释H2S治疗方法振兴了基于H2S的治疗方法的发展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Small
工程技术-材料科学:综合
CiteScore
17.70
自引率
3.80%
发文量
1830
审稿时长
2.1 months
期刊介绍:
Small serves as an exceptional platform for both experimental and theoretical studies in fundamental and applied interdisciplinary research at the nano- and microscale. The journal offers a compelling mix of peer-reviewed Research Articles, Reviews, Perspectives, and Comments.
With a remarkable 2022 Journal Impact Factor of 13.3 (Journal Citation Reports from Clarivate Analytics, 2023), Small remains among the top multidisciplinary journals, covering a wide range of topics at the interface of materials science, chemistry, physics, engineering, medicine, and biology.
Small's readership includes biochemists, biologists, biomedical scientists, chemists, engineers, information technologists, materials scientists, physicists, and theoreticians alike.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


