A General Amino–(Hetero)arylation of Simple Olefins with (Hetero)aryl Sulfonamides Enabled by an N-Triazinyl Group
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
(Hetero)arylethylamines are privileged substructures in pharmaceuticals, agrochemicals, and other bioactive compounds. In principle, the amino–(hetero)arylation of olefins represents an ideal strategy for the rapid preparation of these pharmacophores, which could accelerate the discovery of valuable new products. Established amino–(hetero)arylation methods, however, do not accommodate several important classes of olefins and (hetero)aromatic structures, which precludes access to an appreciable range of molecular architectures. To address these limitations, we have developed a radical-mediated reaction that adds the amino and (hetero)aryl groups from a simple and stable (hetero)aryl sulfonamide across an alkene. The identification of a readily available triazine as an original N-protecting group was critical to the development of this transformation. The reaction features good regio- and stereoselectivity and succeeds with classes of olefins and medicinally valuable (hetero)aryl groups that are unproductive with alternate protocols. Lastly, we highlighted these advances by synthesizing TMP269, a class IIa histone deacetylase inhibitor that would otherwise be challenging to prepare by olefin amino–arylation.
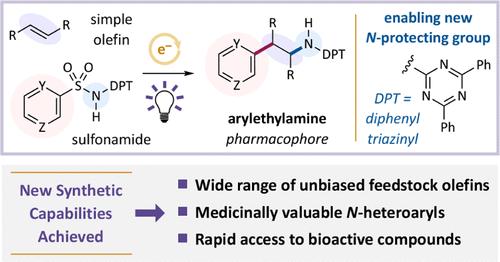
n -三嗪基使简单烯烃与(杂)芳基磺酰胺的一般氨基(杂)芳基化
杂芳基乙胺是药物、农用化学品和其他生物活性化合物的特殊亚结构。原则上,烯烃的氨基(杂)芳基化是快速制备这些药效团的理想策略,可以加速发现有价值的新产品。然而,已建立的氨基(杂)芳基化方法不能适应几种重要的烯烃和(杂)芳结构,这妨碍了获得相当范围的分子结构。为了解决这些限制,我们开发了一个自由基介导的反应,从一个简单和稳定的(杂)芳基磺酰胺中添加氨基和(杂)芳基在烯烃上。鉴定一个现成的三嗪作为原始的n保护基团对这种转变的发展至关重要。该反应具有良好的区域选择性和立体选择性,并且在烯烃类和具有药用价值的(杂)芳基上取得了成功,而在其他方法下则不能产生。最后,我们通过合成TMP269强调了这些进展,TMP269是一种IIa类组蛋白去乙酰化酶抑制剂,否则很难通过烯烃氨基芳基化制备。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


